Treatment of schizophrenia
a treatment method and technology for schizophrenia, applied in the field of treating schizophrenia, can long-term unemployment, poverty and homelessness, antipsychotic drugs fail to significantly improve the negative symptoms and cognitive dysfunction of schizophrenia, and can not solve the problems of accompanied by significant social or occupational dysfunction,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
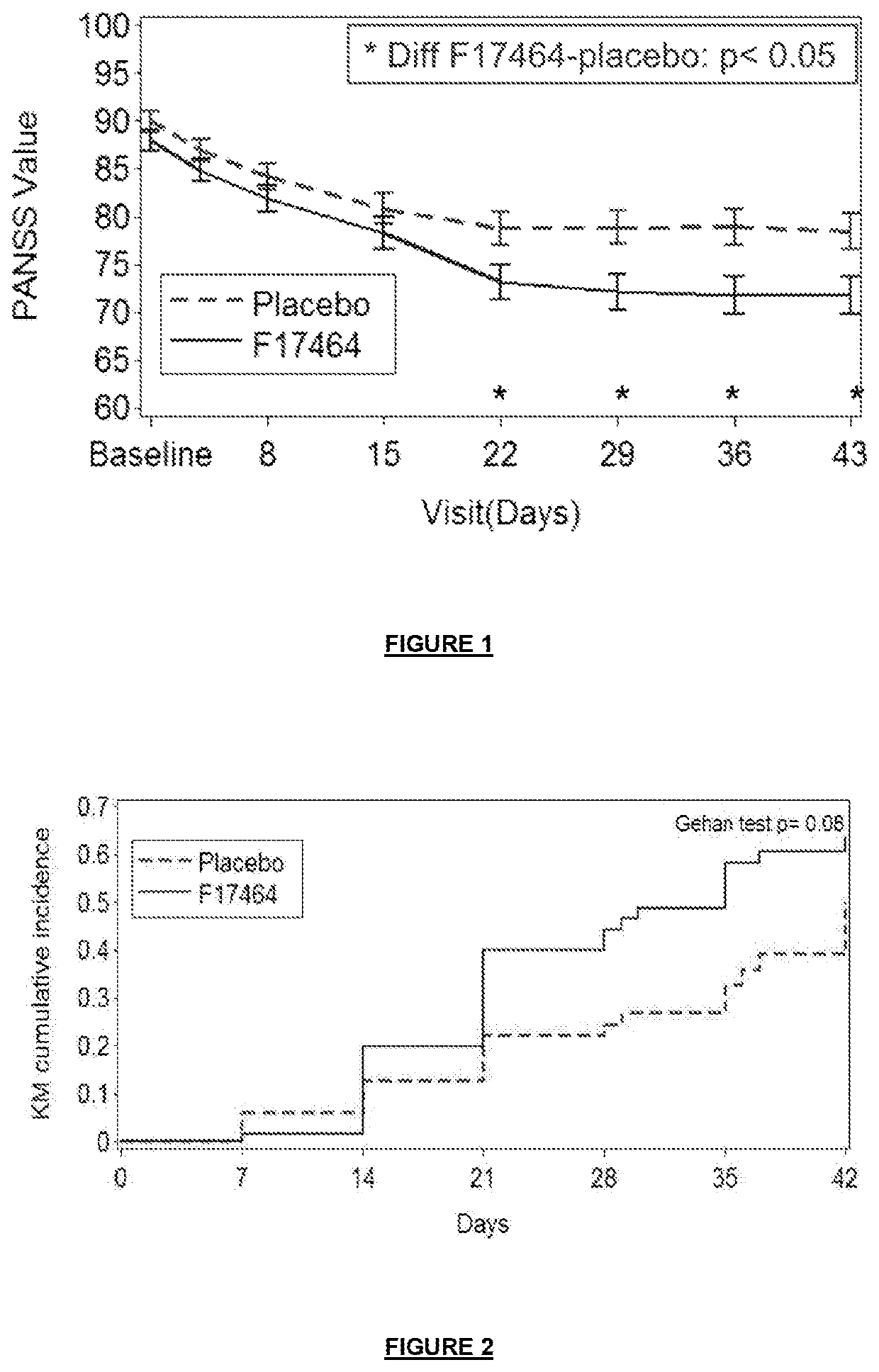
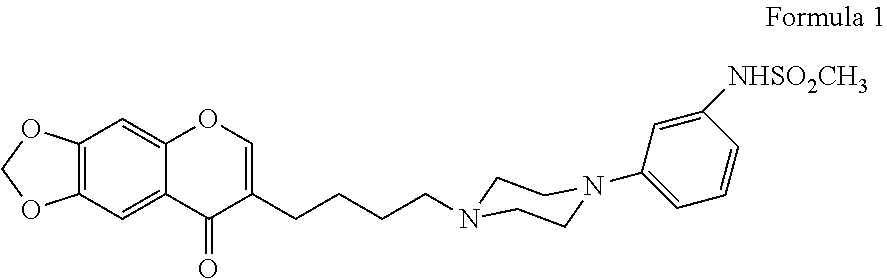
of F17464 Clinical Study Results
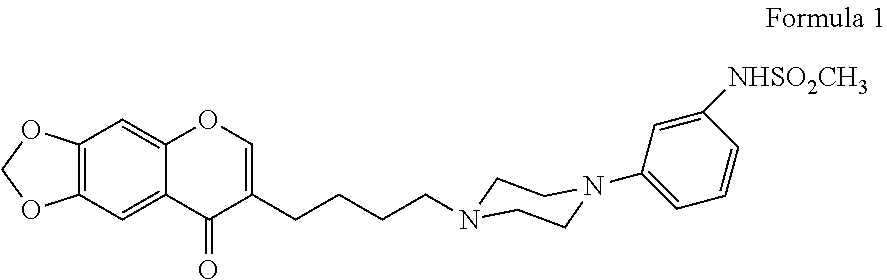
[0198]N-(3-{4-[4-(8-oxo-8H-[1,3]dioxolo[4,5-g]chromen-7-yl)-butyl]-piperazin-1-yl}-phenyl)-methanesulfonamide hydrochloride has been tested in a placebo-controlled study of patients with an acute exacerbation of schizophrenia.
[0199]2.1 Methods:
[0200]This double-blind, parallel group, multicenter study included patients with acute exacerbation of schizophrenia treated either with F17464 fixed dose 40 mg (20 mg bid) or placebo (randomization 1:1) for 6 weeks as antipsychotic monotherapy. The primary objective was to evaluate the efficacy of F17464 in comparison to placebo. The primary efficacy criterion was the PANSS (Positive and Negative Syndrome Scale) total score change from baseline to Day 43 on the Full Analysis Set (FAS).
[0201]Inclusion criteria included a well-documented diagnosis of schizophrenia for a minimum of 1 year, and a recent acute exacerbation characterized by a PANSS total score at screening ≥70 and <120 with no significant change of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com