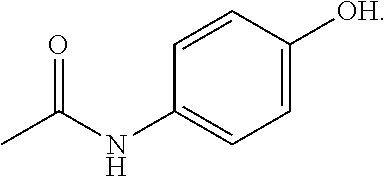
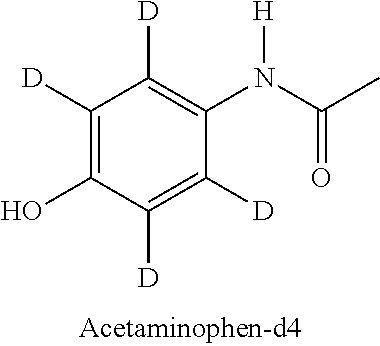
Deuterated forms of acetaminophen and uses thereof
a technology of acetaminophen and deuterated forms, which is applied in the field of deuterated forms of acetaminophen and, can solve the problems of acetaminophen overdose remains a significant problem, the safety of acetaminophen is not known, and scientists have been unsuccessful in developing and commercializing a safer, improved form of acetaminophen, so as to avoid or reduce the incidence of life-threatening side effects, differen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
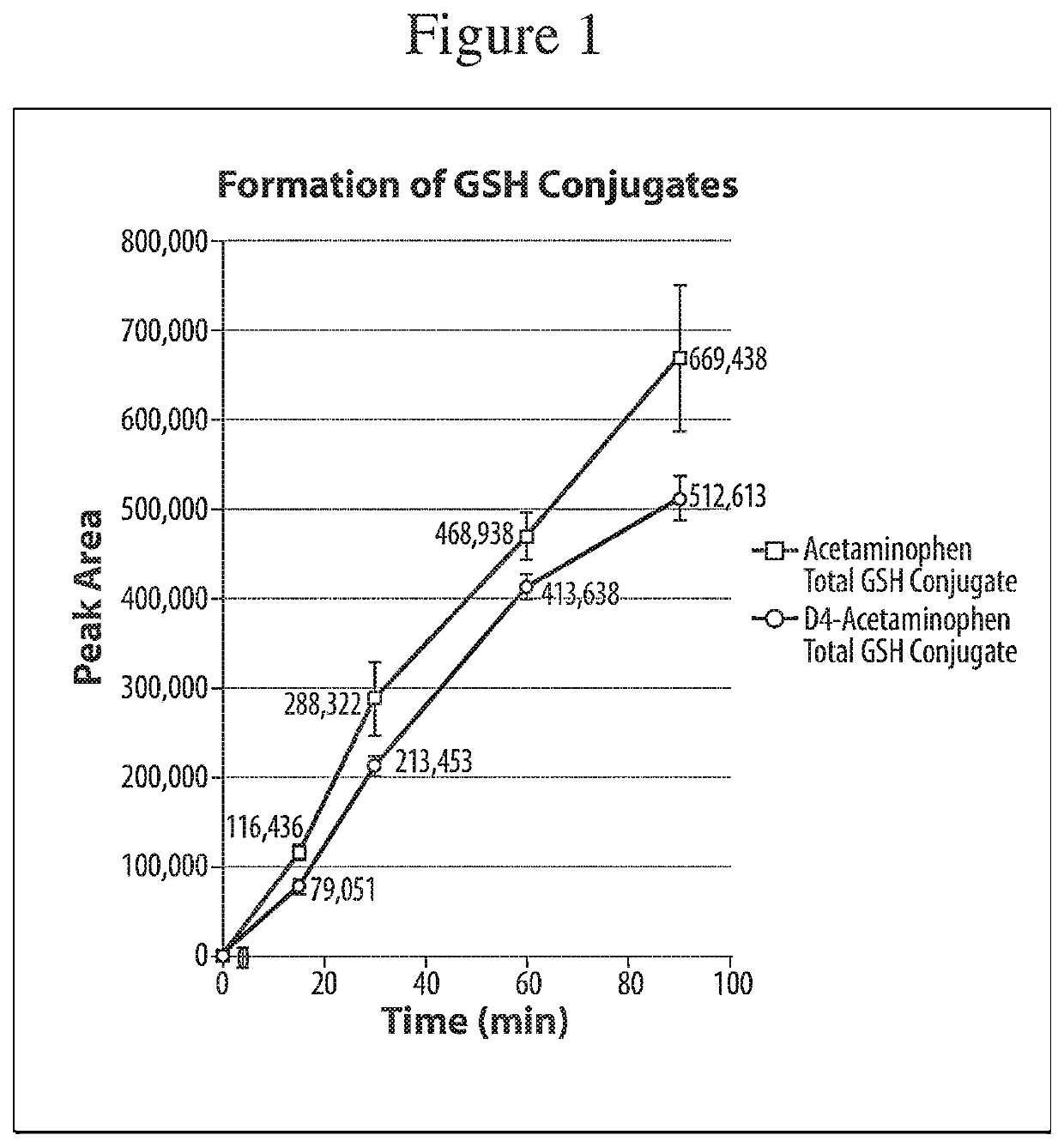
[0059]A study was performed to assess whether a deuterated form of acetaminophen would reduce the production of the reactive metabolite NAPQI when compared to a non-deuterated form of acetaminophen. In the study, acetaminophen and acetaminophen-d4 were incubated with human liver microsomes, with glutathione (GSH) as a trapping agent. The collected samples were analyzed by UPLC / HR-MS to screen and identify the possibly forming trapped reactive metabolites.[0060]Sample type: Pooled liver microsomes[0061]Species: Human (pooled)[0062]Time points: 0, 15, 30, 60, 90 minutes with and without cofactors (all with 2 mM GSH)+60 minutes blank[0063]Concentration: 10 μM[0064]Cofactors: NADPH (2 mM), UDPGA (1 mM), alamethicin 15 μg / ml[0065]Protein content: 1 mg / ml[0066]Replicates: 3[0067]Buffer: phosphate buffer pH 7.4+MgCl2 (2 mM)[0068]Incubation volume: 300 μl; Sampling volume: 40 μl; Quenching: 2-fold volume of 75% acetonitrile[0069]Stock solvent: 50% DMSO (spiking 1 / 100 to incubation)[0070]Pos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| natural abundance | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com