Synthetic transmembrane components
a technology of transmembrane and components, applied in the direction of fusion polypeptides, bacteria, fungi, etc., can solve the problems of limiting the therapeutic potential of this approach, prone to signalling, and inability to guarantee the success of this approach, and achieve the effect of long shelf life in storag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Cloning Cassette System
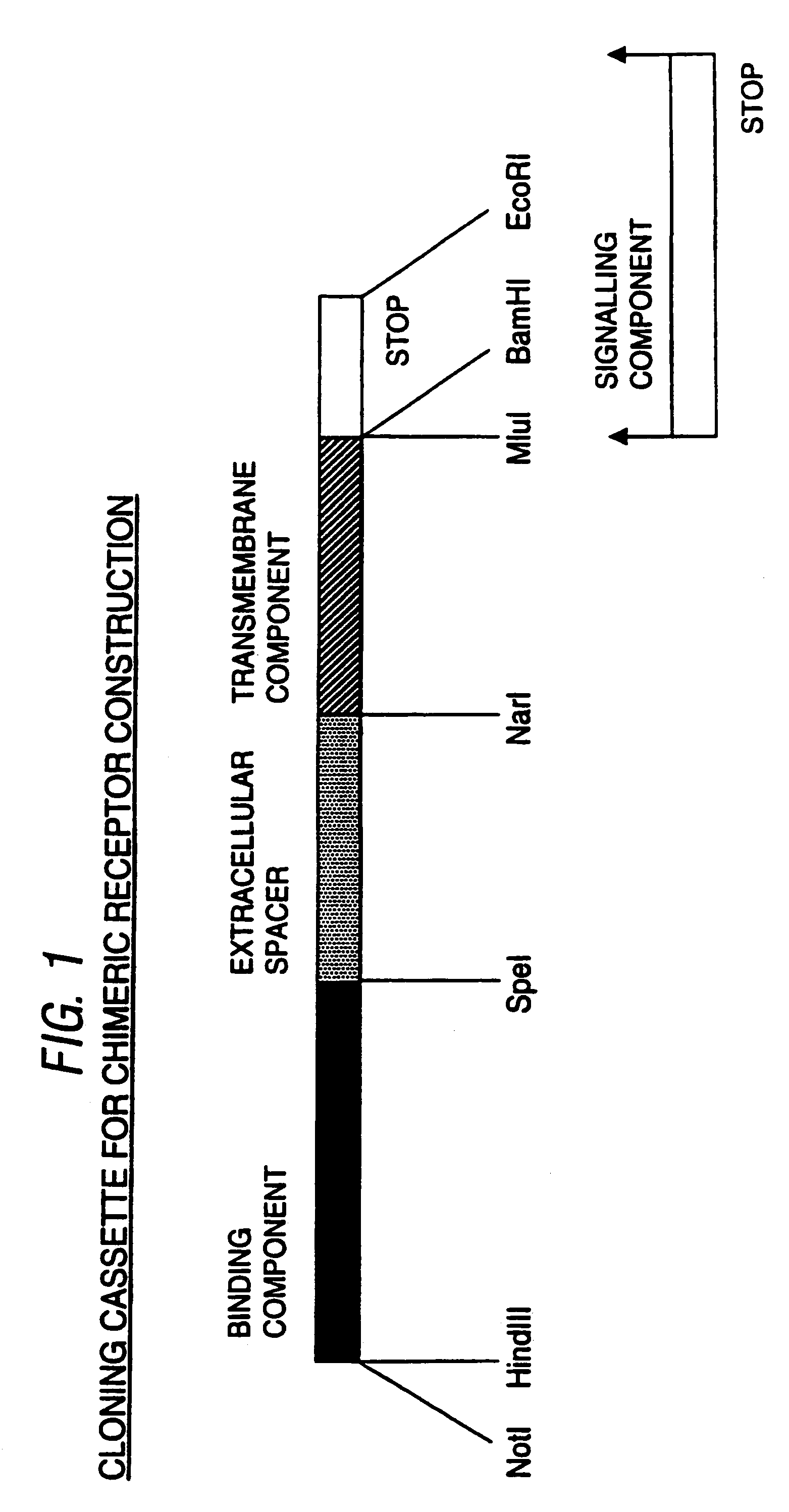
[0092]To facilitate construction of chimeric receptors with different binding, extracellular spacer, transmembrane and signalling components, a cloning cassette system was devised in pBluescript SK+ (Stratagene). This is a modification of our cassette system described in International Patent Specification No. WO97 / 23613.
[0093]This new cassette system is shown in FIG. 1. The binding component has 5′ Not I and Hind III restriction sites and a 3′ Spe I restriction site. The extracellular spacer has a 5′ Spe I site (Thr, Ser) and a 3′ Nar I site (Gly, Ala). The transmembrane component has a 5′ Nar I site (Gly, Ala) and 3′ Mlu I (Thr, Arg) and BamHI sites (Gly, Ser). The signalling component has a 5′ BamHI site and a 3′ EcoRI site. In between this BamHI and EcoRI site is a stop codon for receptors without a signalling component.
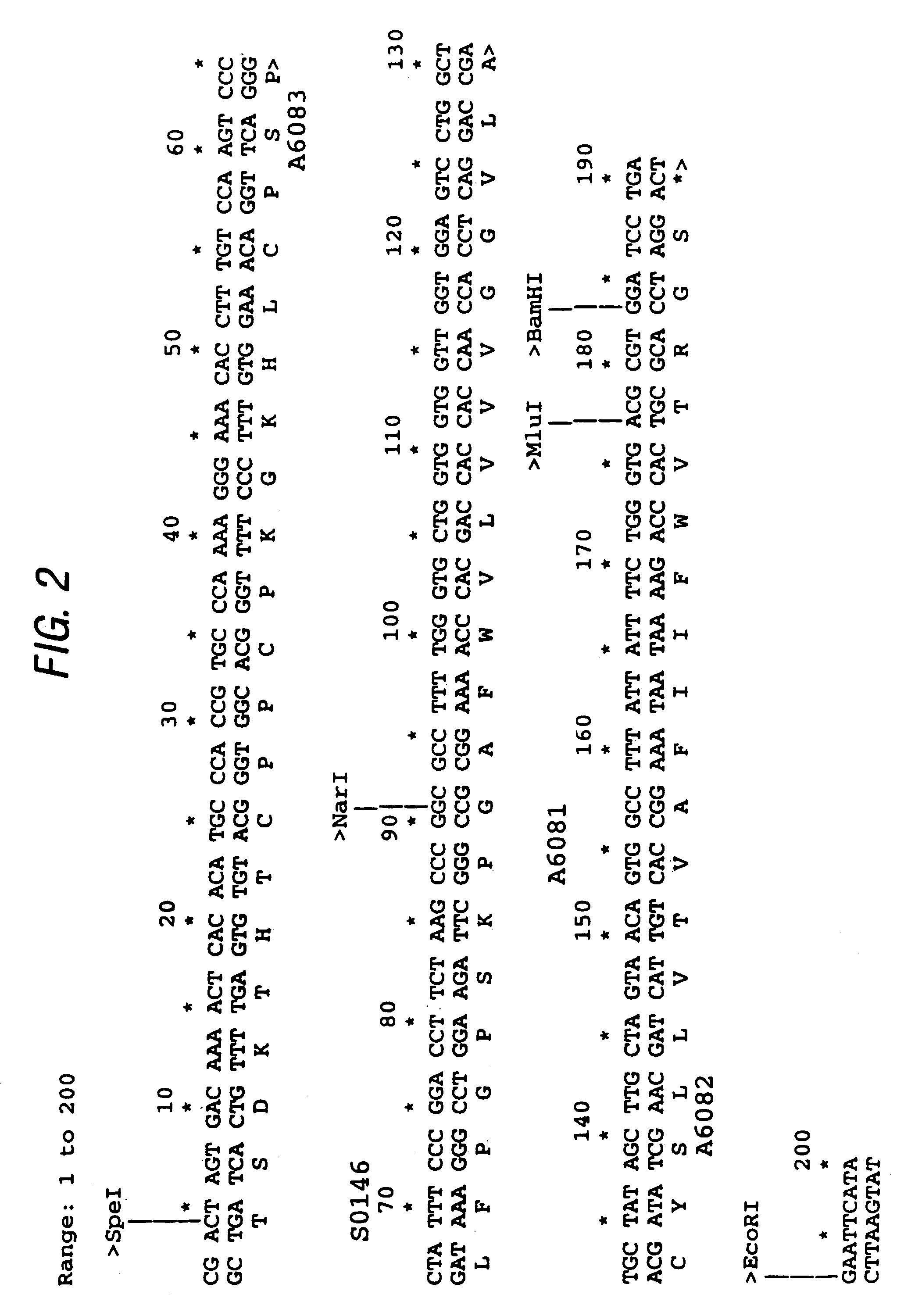
[0094]To generate this cassette, a 200 bp fragment was PCR assembled using oligos:—S0146 (SEQ ID NO:3), A6081 (SEQ ID NO:4)...
example 2
Construction of Chimeric Receptors With Different Transmembrane Components
a) P67scFv / h.CD28 / CD28Tm / FcRγChimeric Receptor
[0095]This construct was generated from the cassette described above and forms the basis for chimeric receptor constructs (b) to (f). The FcRγ intracellular component was PCR cloned with oligos A9515 (SEQ ID NO:7) and A9516 (SEQ ID NO:8) (FIG. 3) from human Leukocyte cDNA (Clontech) and cloned into the BamHI site of the described cassette (FIG. 1).
[0096]The binding component, P67 single chain Fv (scFv) with specificity for CD33 and CD33 on HL60 cells, consists of a human antibody leader sequence and the variable component of the light chain of the engineered human antibody linked via a (Gly4Ser)5 (SEQ ID NO:26) linker to the variable component of the heavy chain of the engineered human antibody. This binding component is described in WO 97 / 23613. The extracellular spacer component h.CD28, consists of residues 234 to 243 of human IgG1 hinge and residues 118 to 134 o...
example 3
Construction of Recruitment Receptors With Different Transmembrane Components
a) P67scFv / h.CD28 / CD28Tm.stop Recruitment Receptor
[0108]This construct was generated as described for the cloning cassette and forms the basis for subsequent recruitment receptor constructs (FIGS. 1 and 2).
[0109]The binding component, P67 single chain Fv (scFv) consists of a human antibody leader sequence and the variable component of the light chain of the engineered human antibody linked via a (Gly4Ser)5 (SEQ ID NO:26) linker to the variable component of the heavy chain of the engineered human antibody. This binding component is described in WO 97 / 23613. The extracellular spacer component h.CD28, consists of residues 234 to 243 of human IgG1 hinge and residues 118 to 134 of human CD28. The transmembrane component consists of residues 135 to 161 of human CD28 (A. Aruffo & B. Seed 1987 PNAS84 8573–8577). This is followed by an in frame stop codon.
b) P67scFv / h.CD28 / Tm20.stop Recruitment Receptor
[0110]This re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com