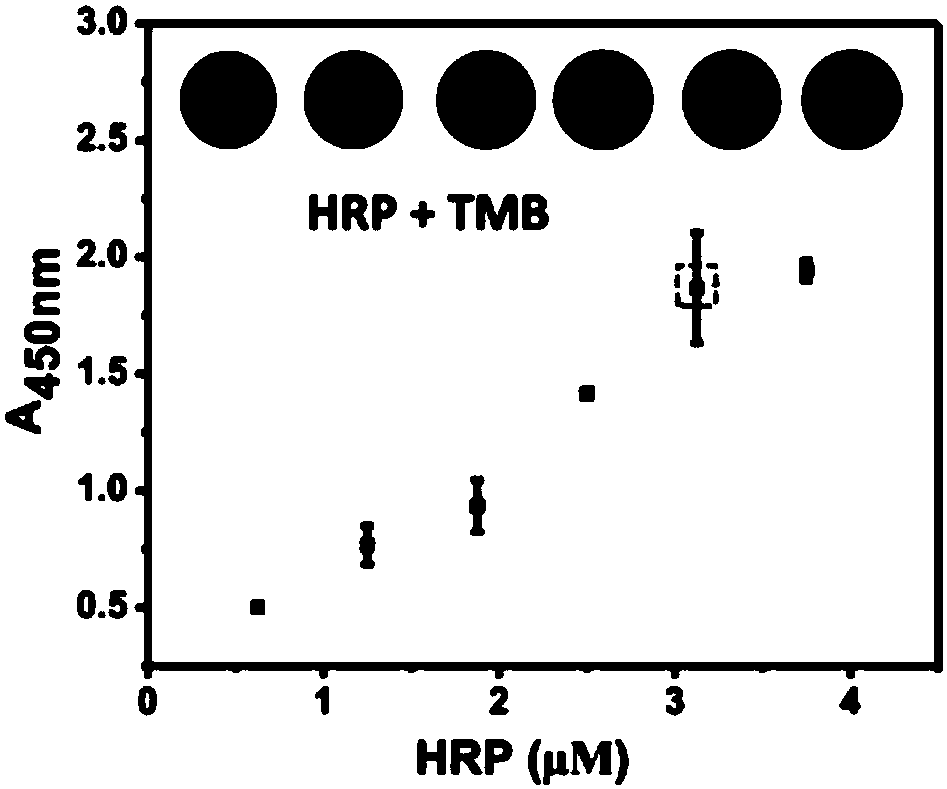
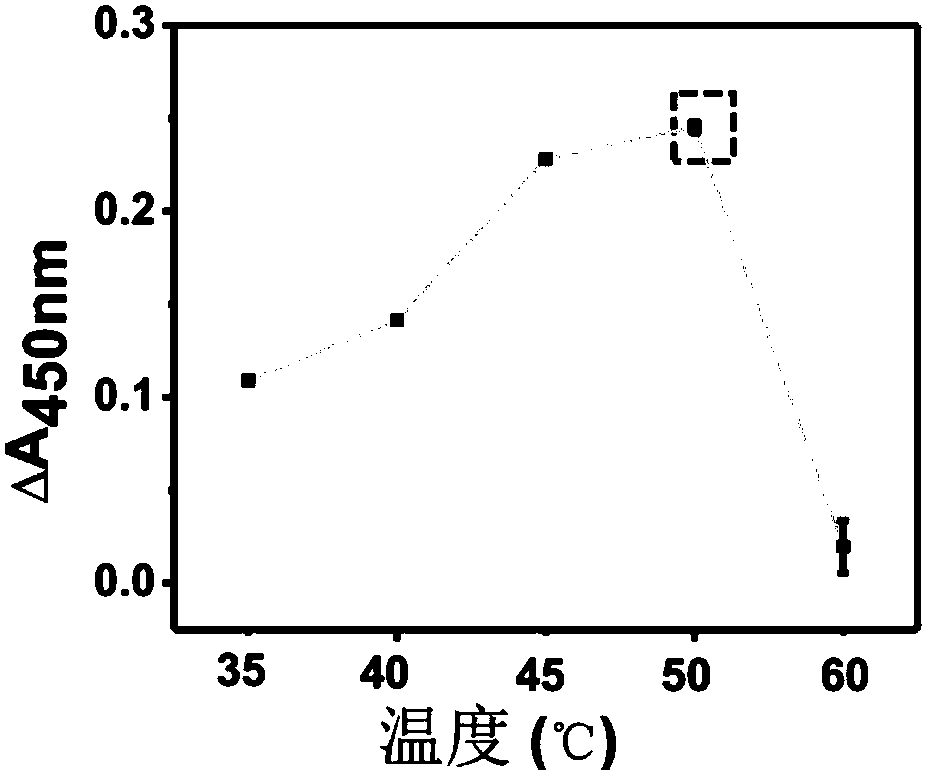
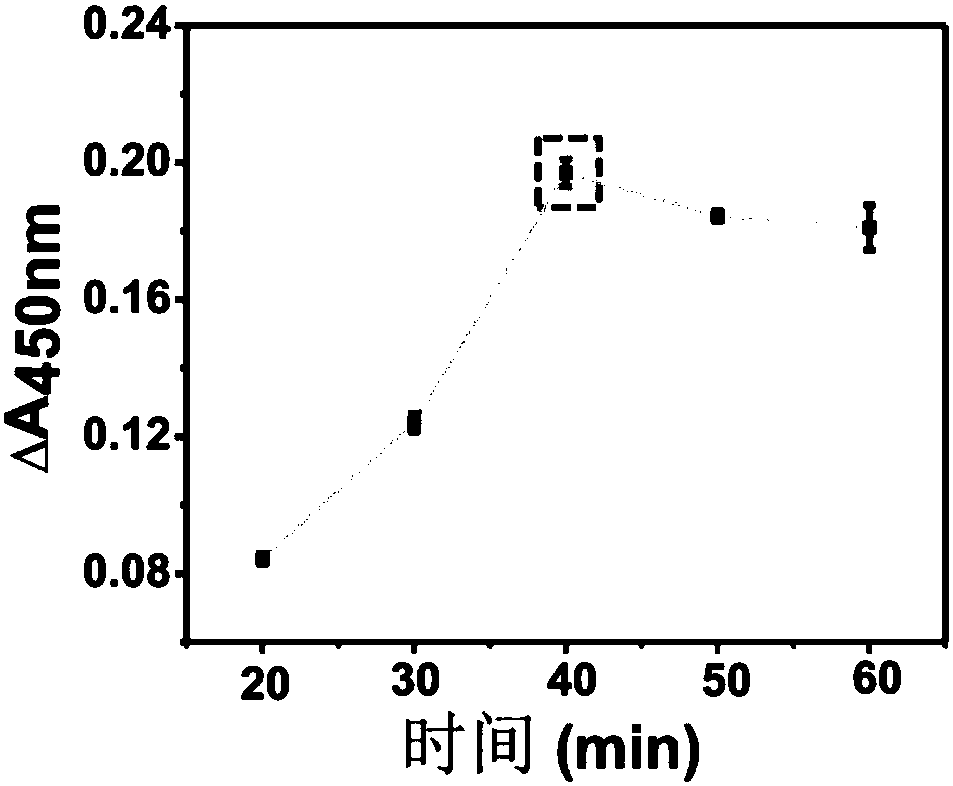
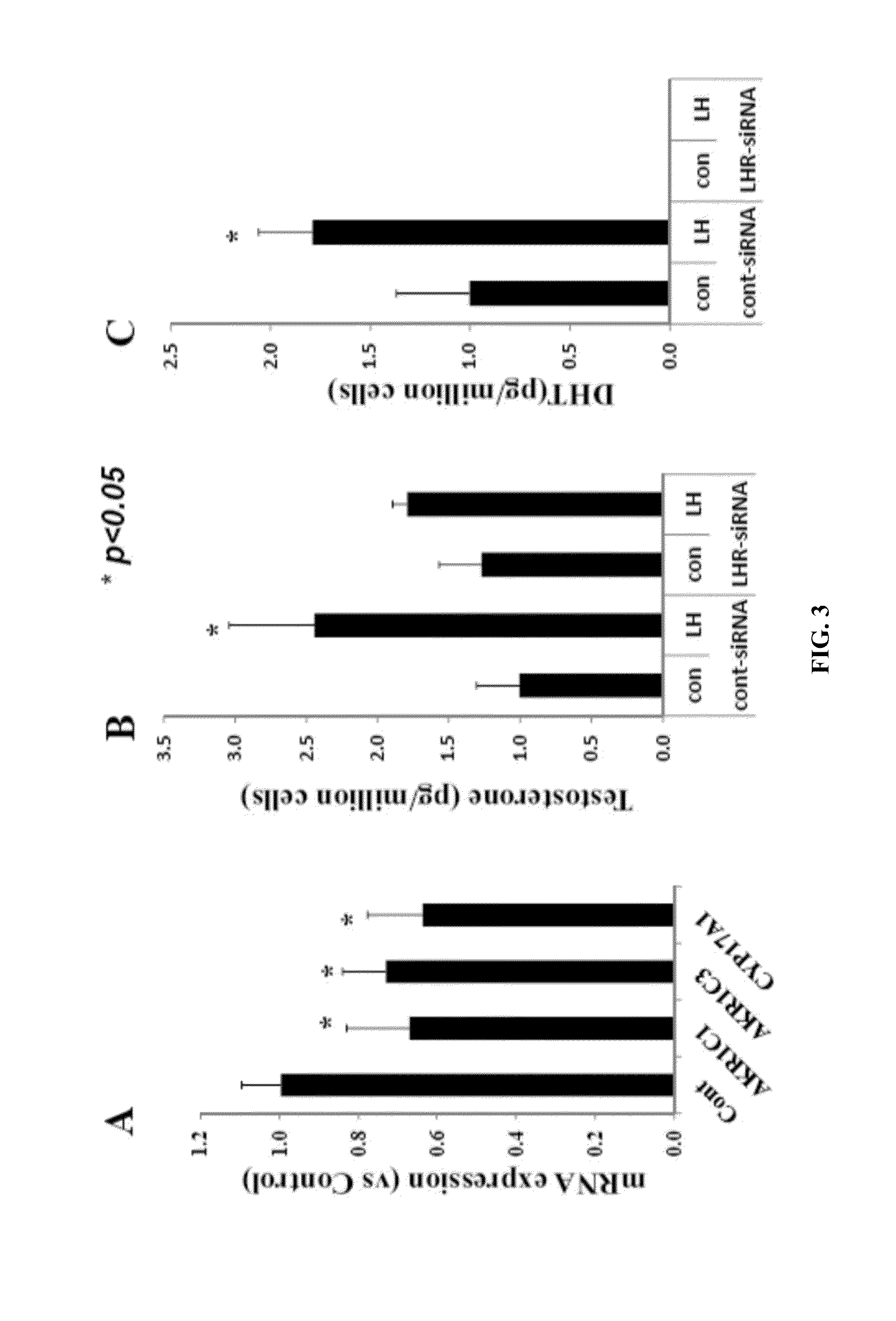
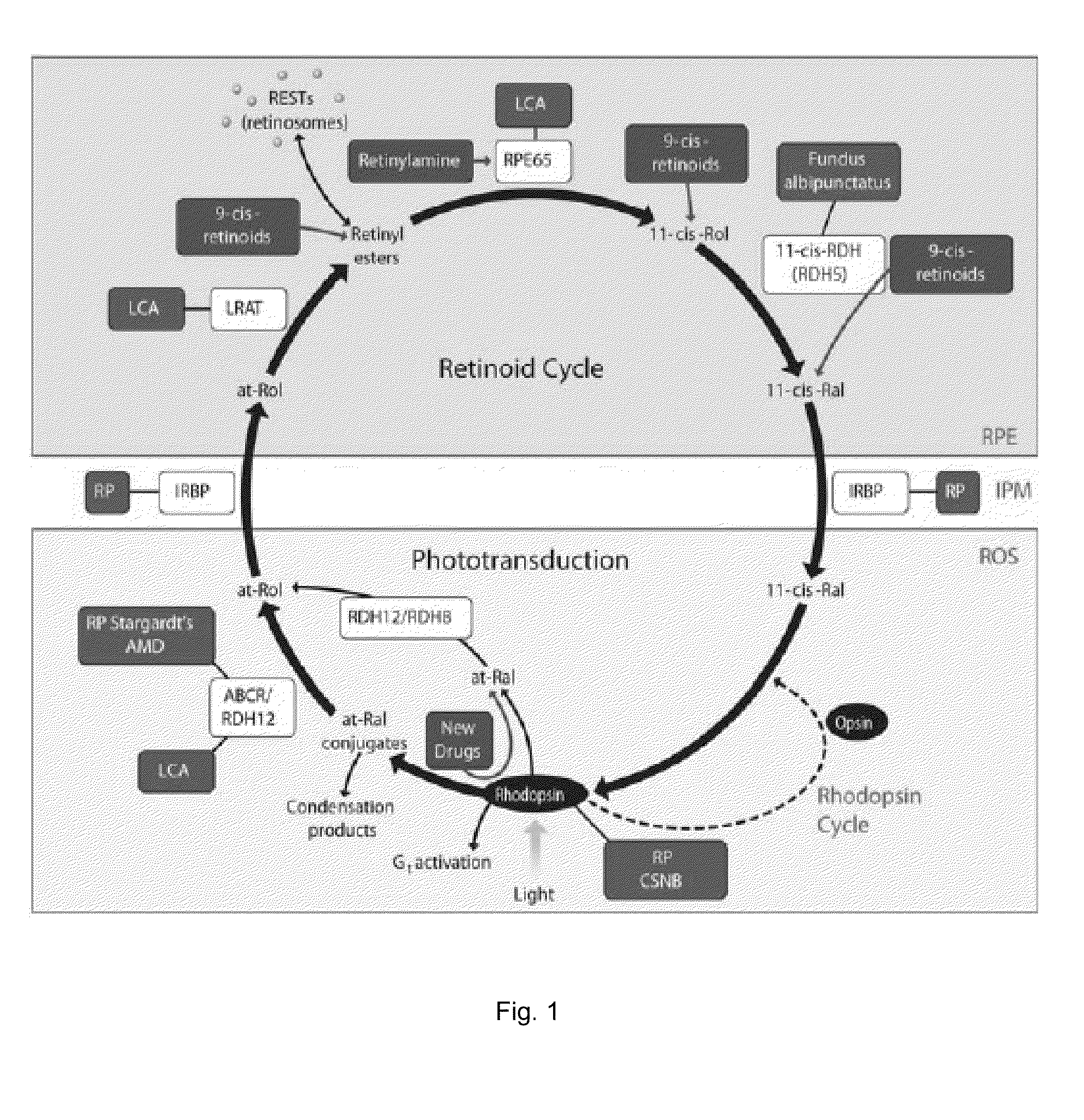
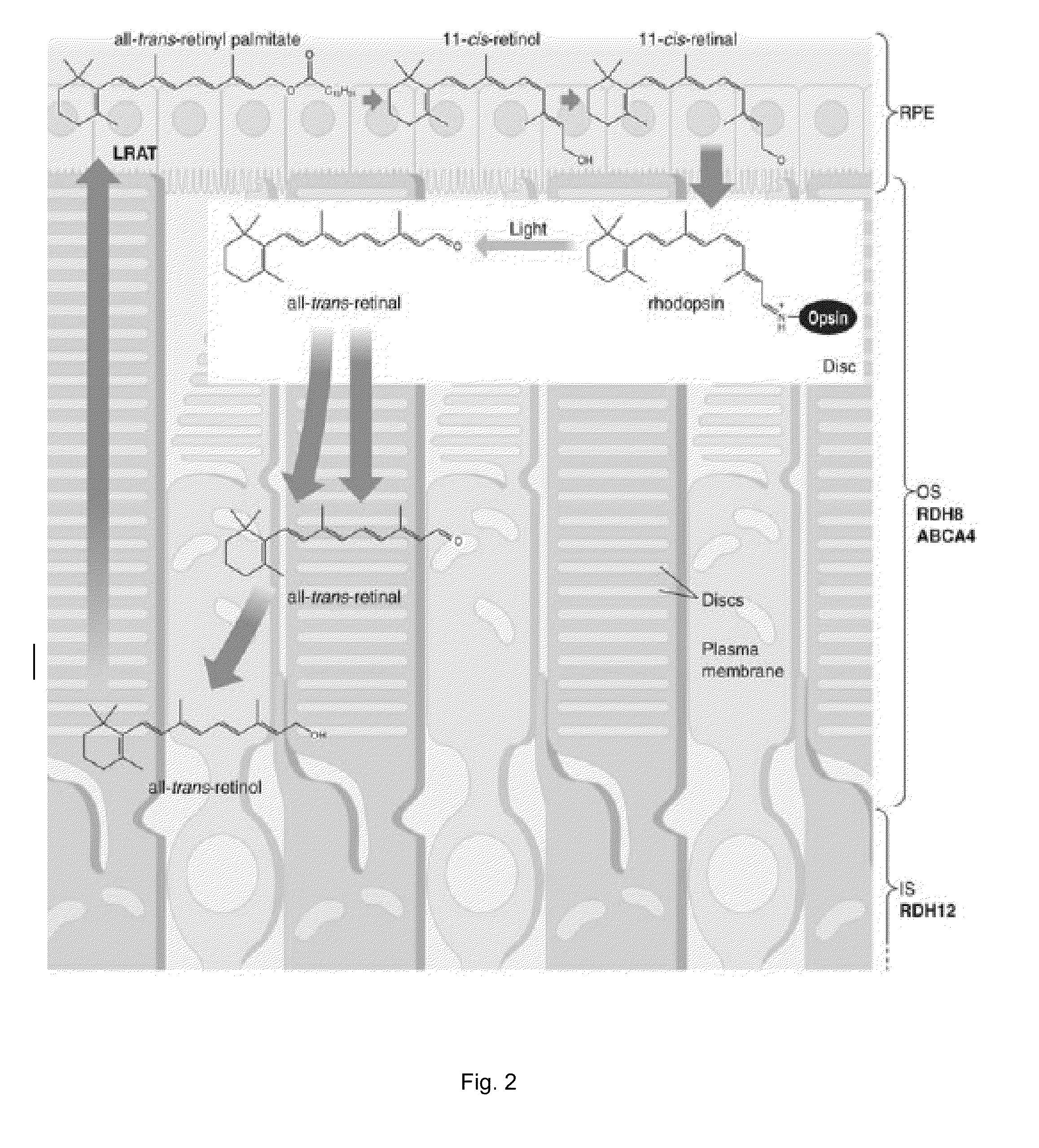
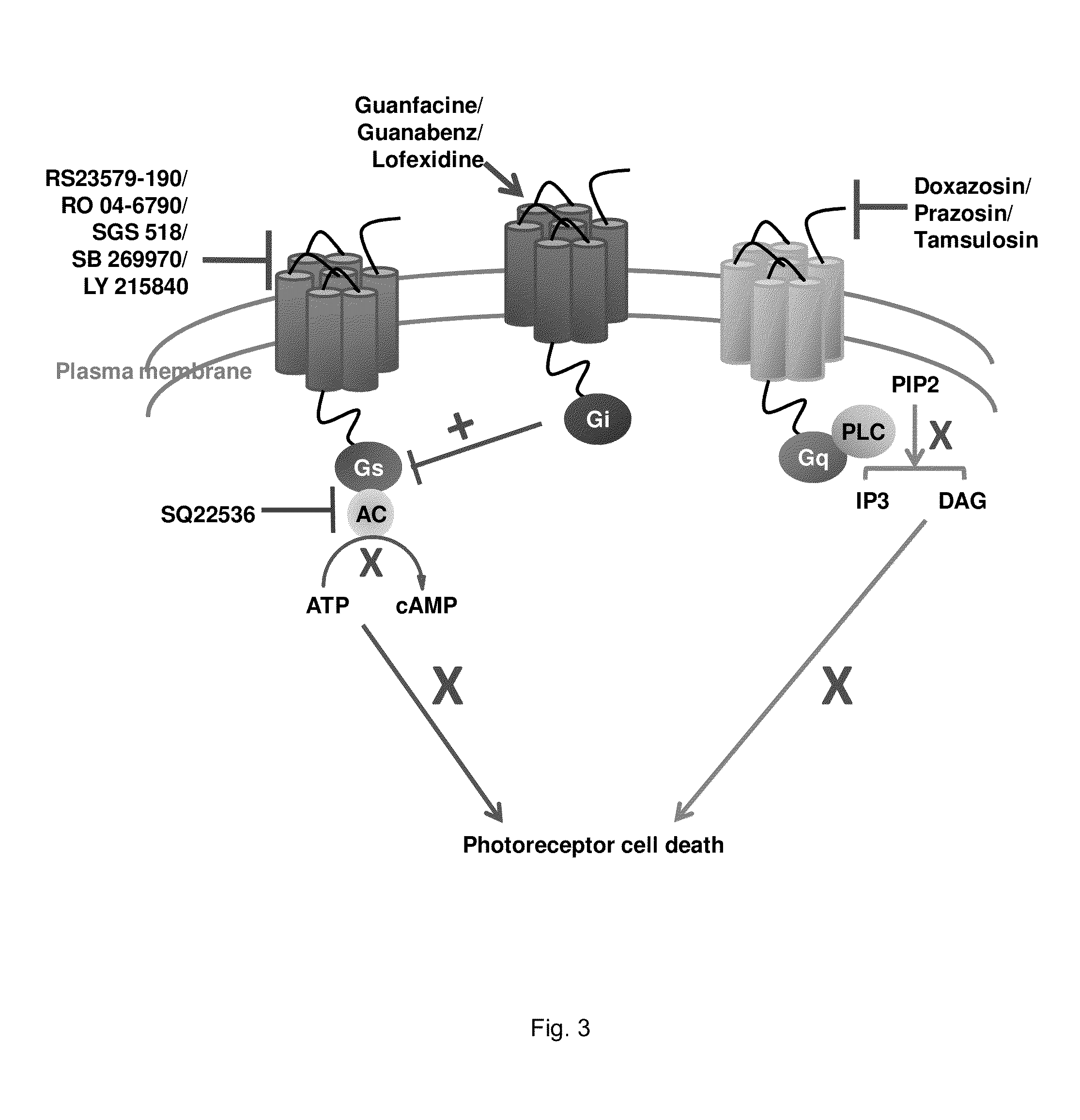
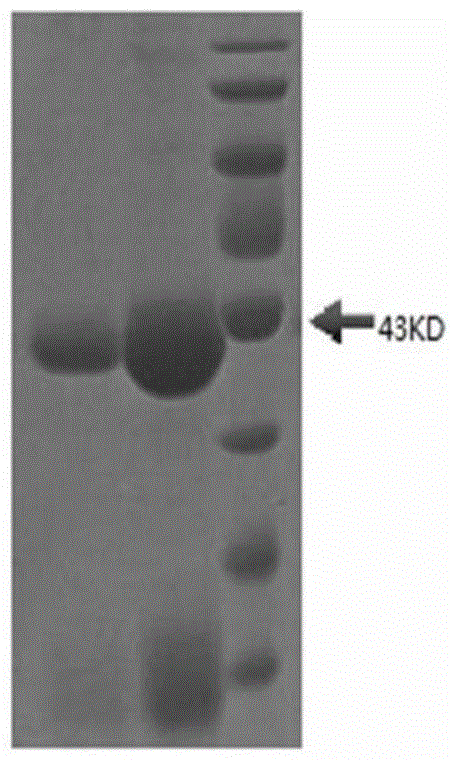
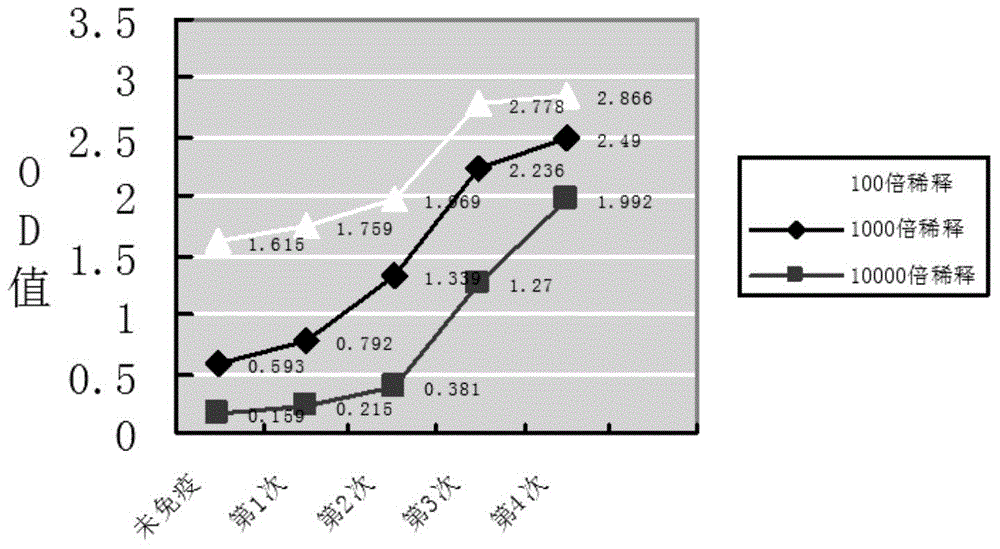
Patents
Literature
72 results about "Signalling cascade" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
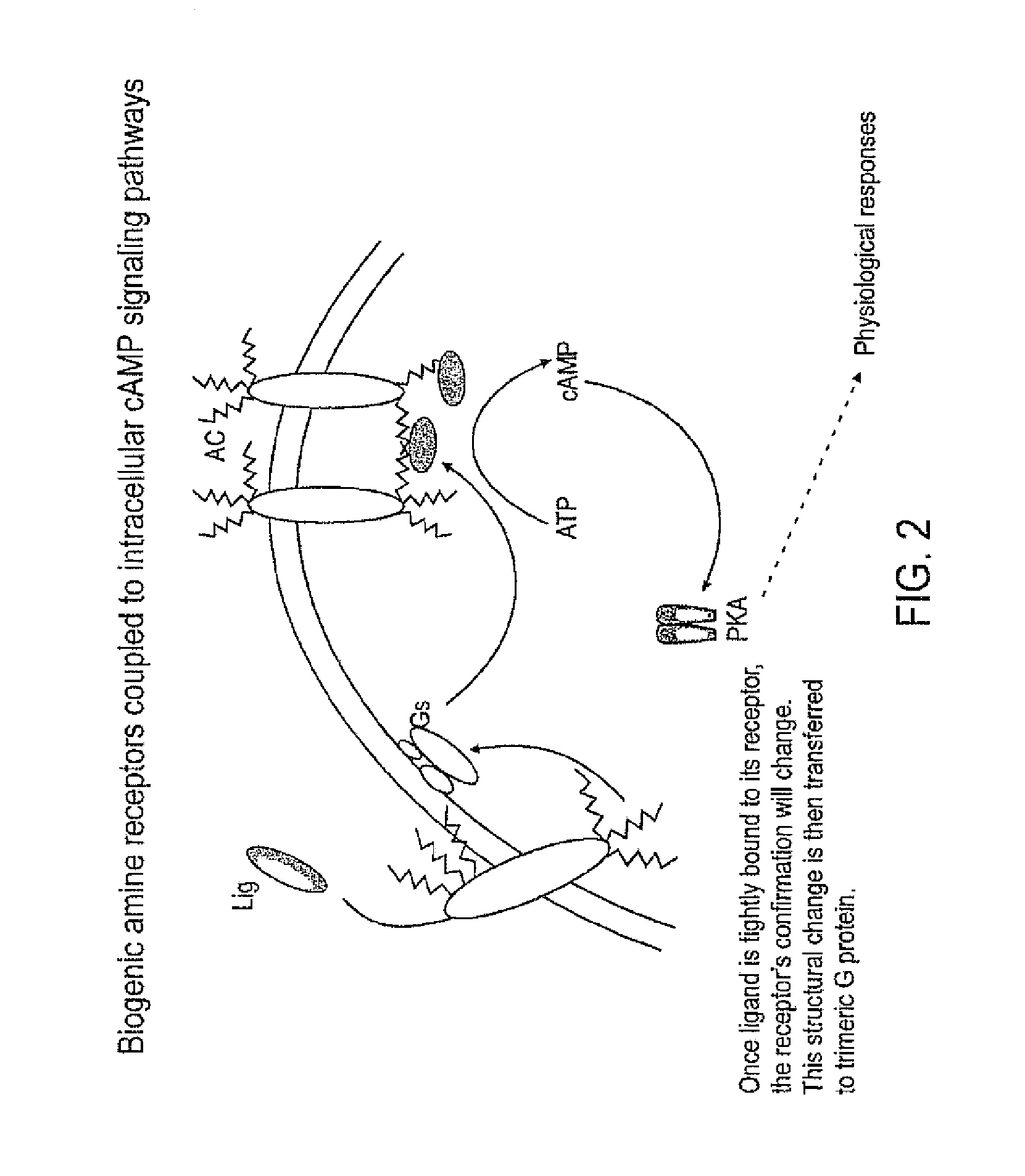
A biochemical cascade, also known as a signaling cascade or signaling pathway, is a series of chemical reactions which are initiated by a stimulus (first messenger) acting on a receptor that is transduced to the cell interior through second messengers (which amplify the initial signal) and ultimately to effector molecules, resulting in a cell ...
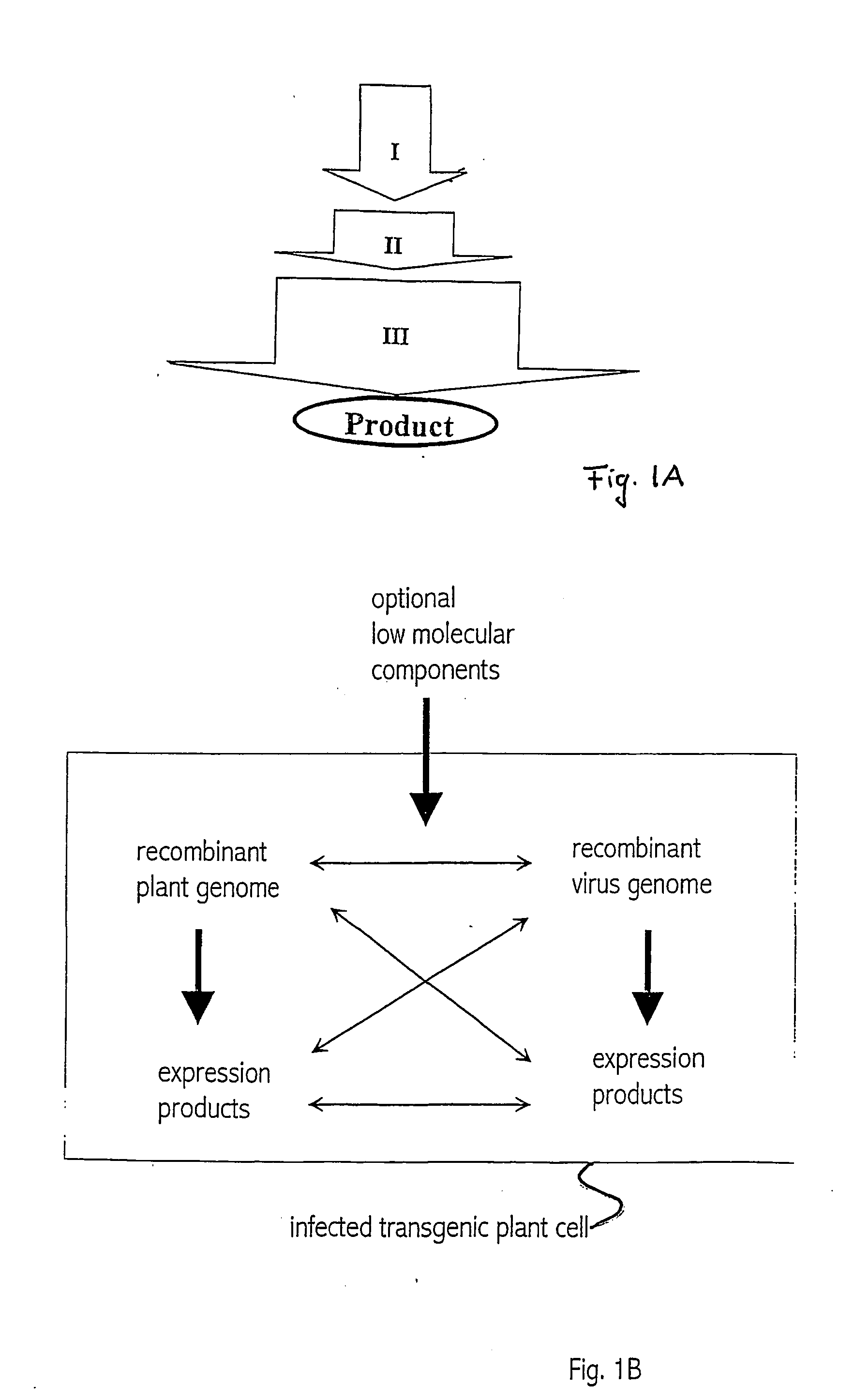
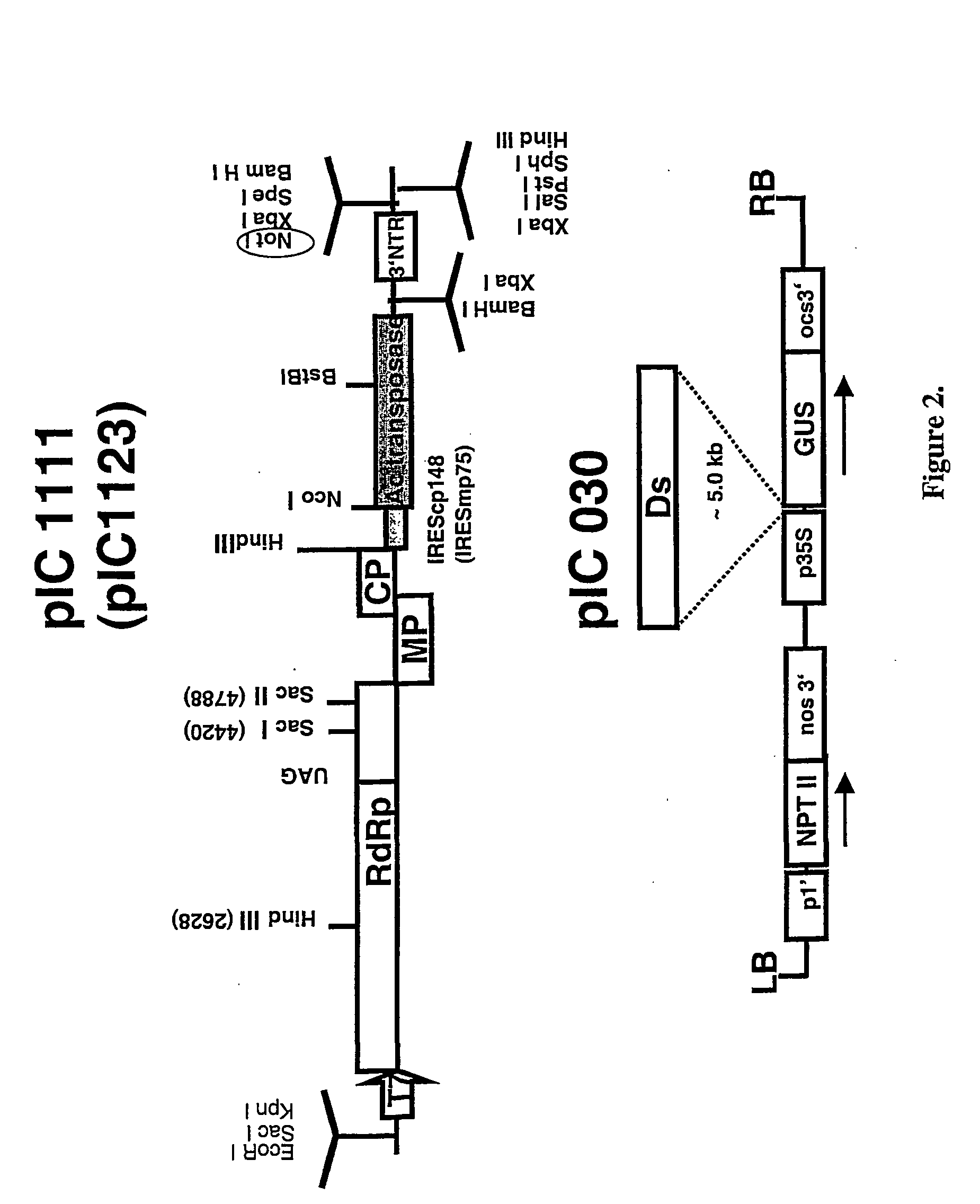
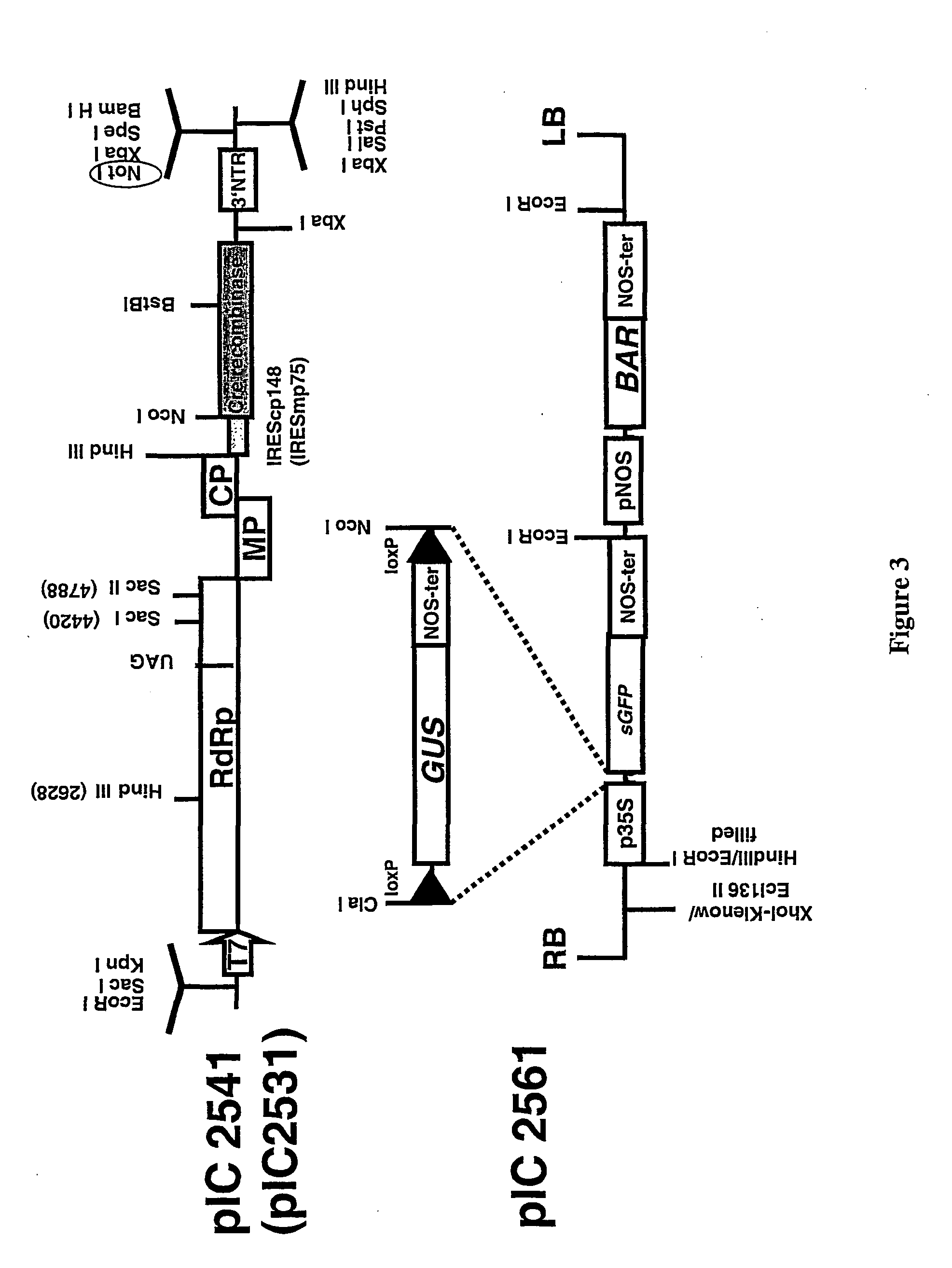
Recombinant viral switches for the control of gene expression in plants
InactiveUS20050091706A1Improve interferenceGrowth retardationOther foreign material introduction processesFermentationHeterologousPlant virus
Owner:ICON GENETICS
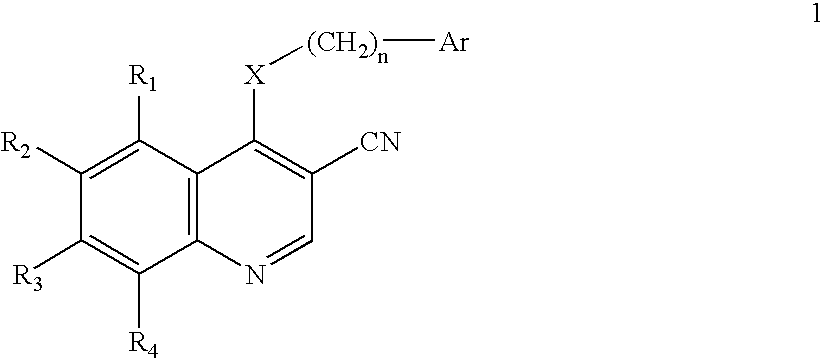
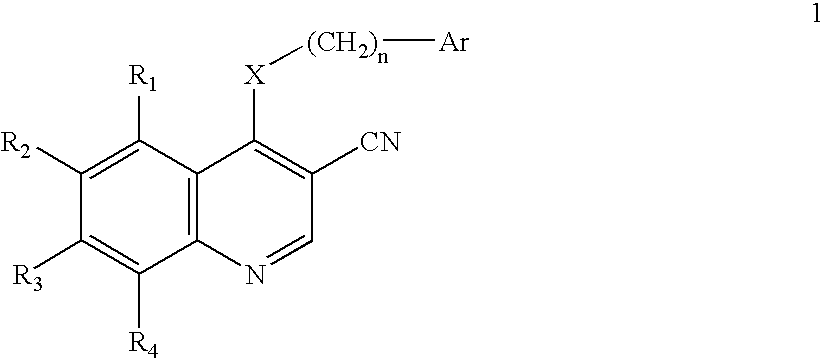
3-Quinolinecarbonitrile protein kinase inhibitors
This invention provides a compound of Formula 1 where Ar, X, R1, R2, R3, and R4 are defined herein, or a pharmaceutically acceptable salt thereof useful in the prevention or inhibition of diseases associated with the Ras / Raf / MEK signaling cascade in a mammal, such as neoplasms, strokes, osteoporosis, cancer, rheumatoid arthritis, inflammatory disease, polycystic kidney disease, and colonic polyps, and methods of making the compounds of formula 1 and intermediates.
Owner:WYETH
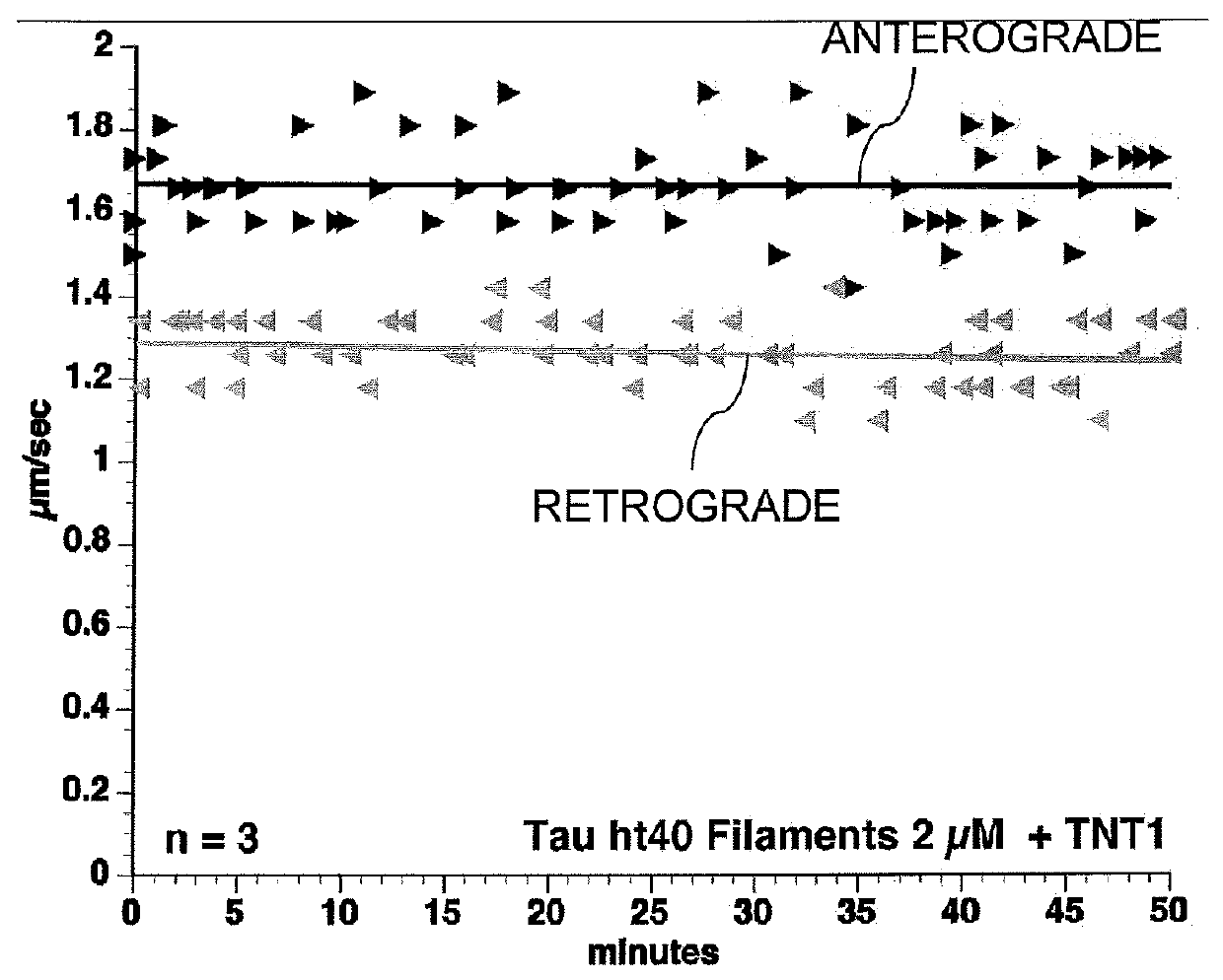
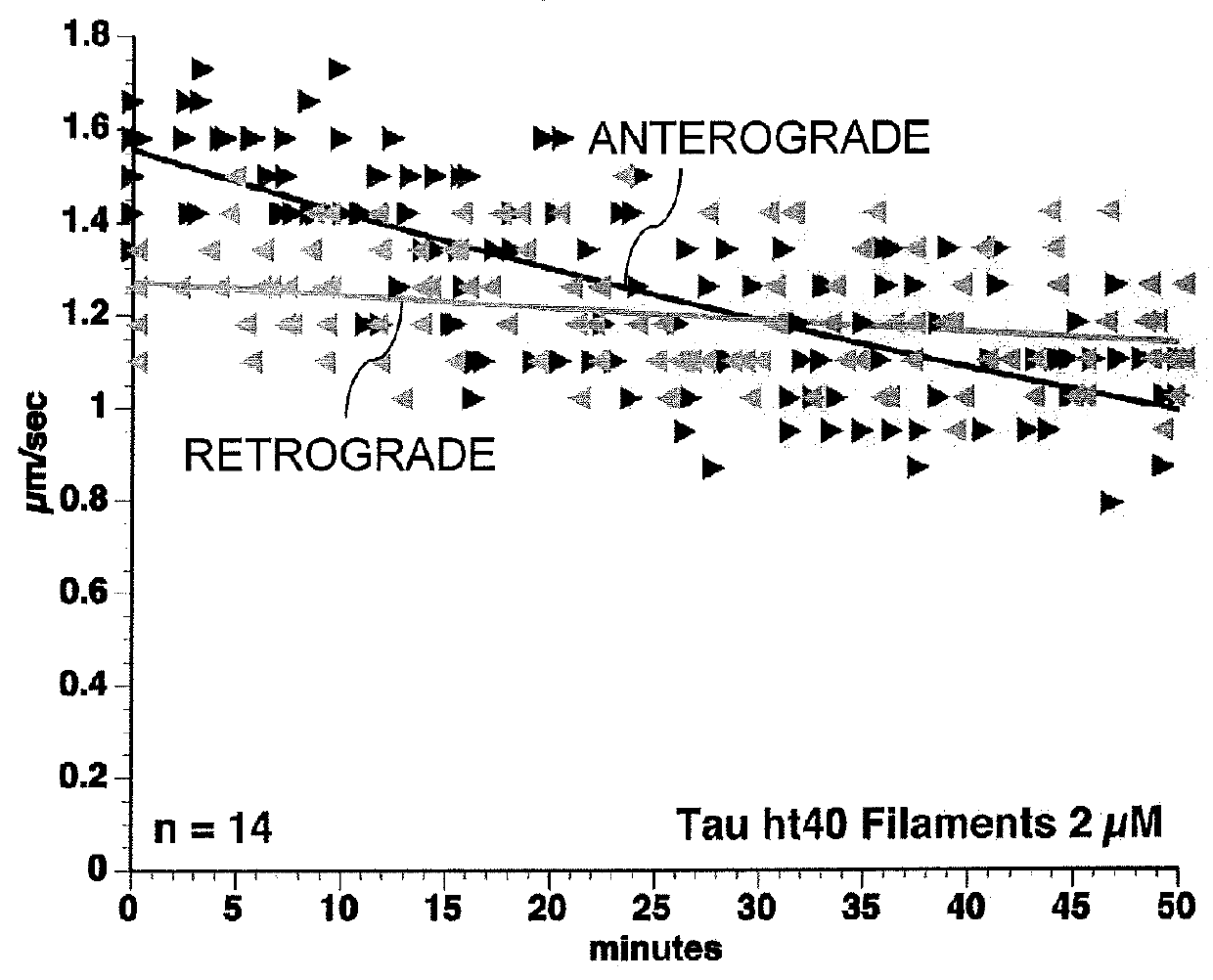
Composition and Method for Preventing or Treating a Tauopathy
ActiveUS20120142602A1Nervous disorderPeptide/protein ingredientsBiological activationSignalling cascade
The present invention is a composition and method for the prevention and treatment of a tauopathy. The composition of the invention includes N-terminal amino acid residues of the tau protein, which have been identified as being involved in toxic activation of a PP1 / GSK3 signaling cascade and inhibition of fast axonal transport in human tauopathies.
Owner:NORTHWESTERN UNIV +1
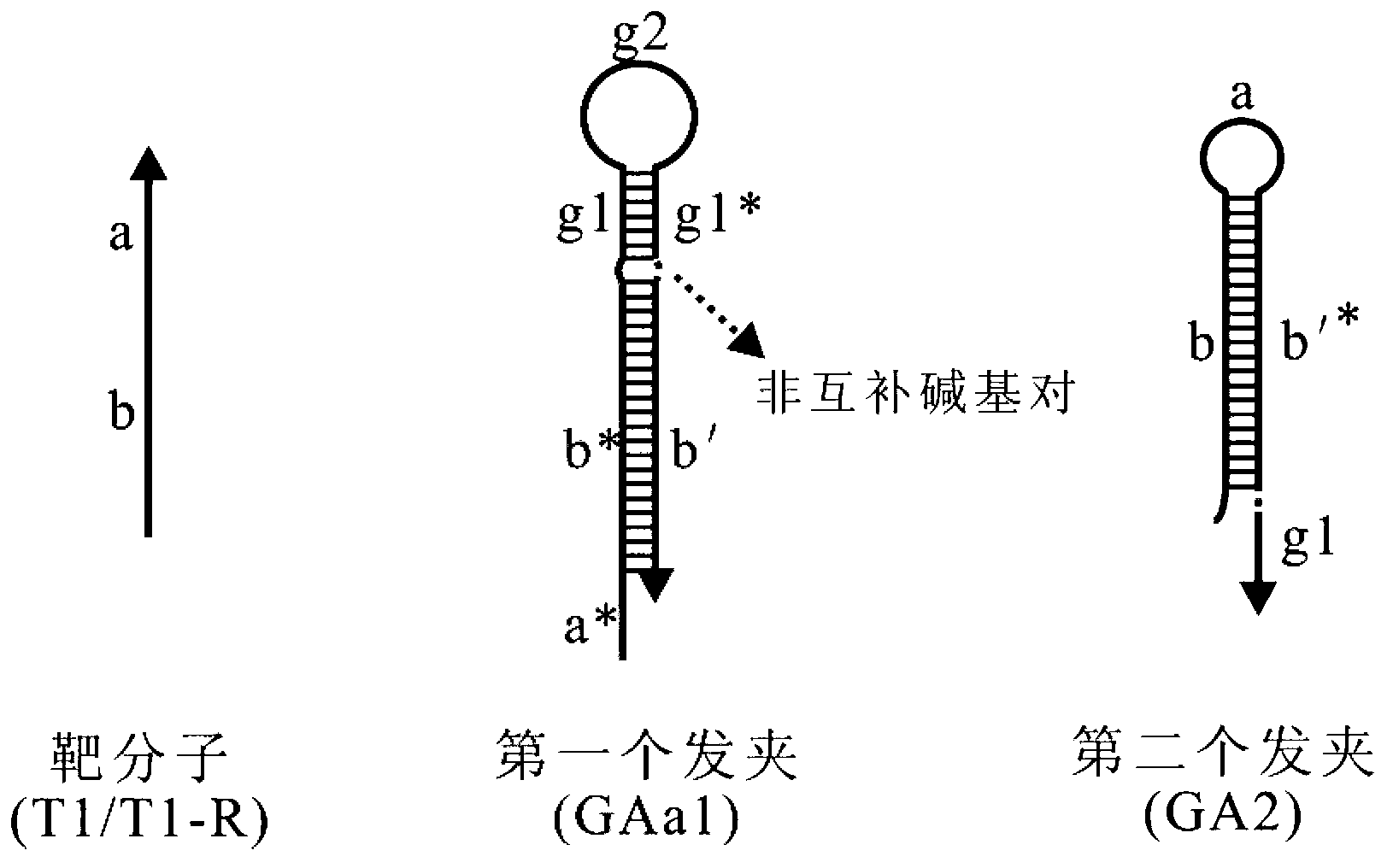
Oligonucleotide probe, and method for detecting target molecule through using it
ActiveCN102827836ARealize quantitative detectionReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationSequence designNucleic Acid Probes
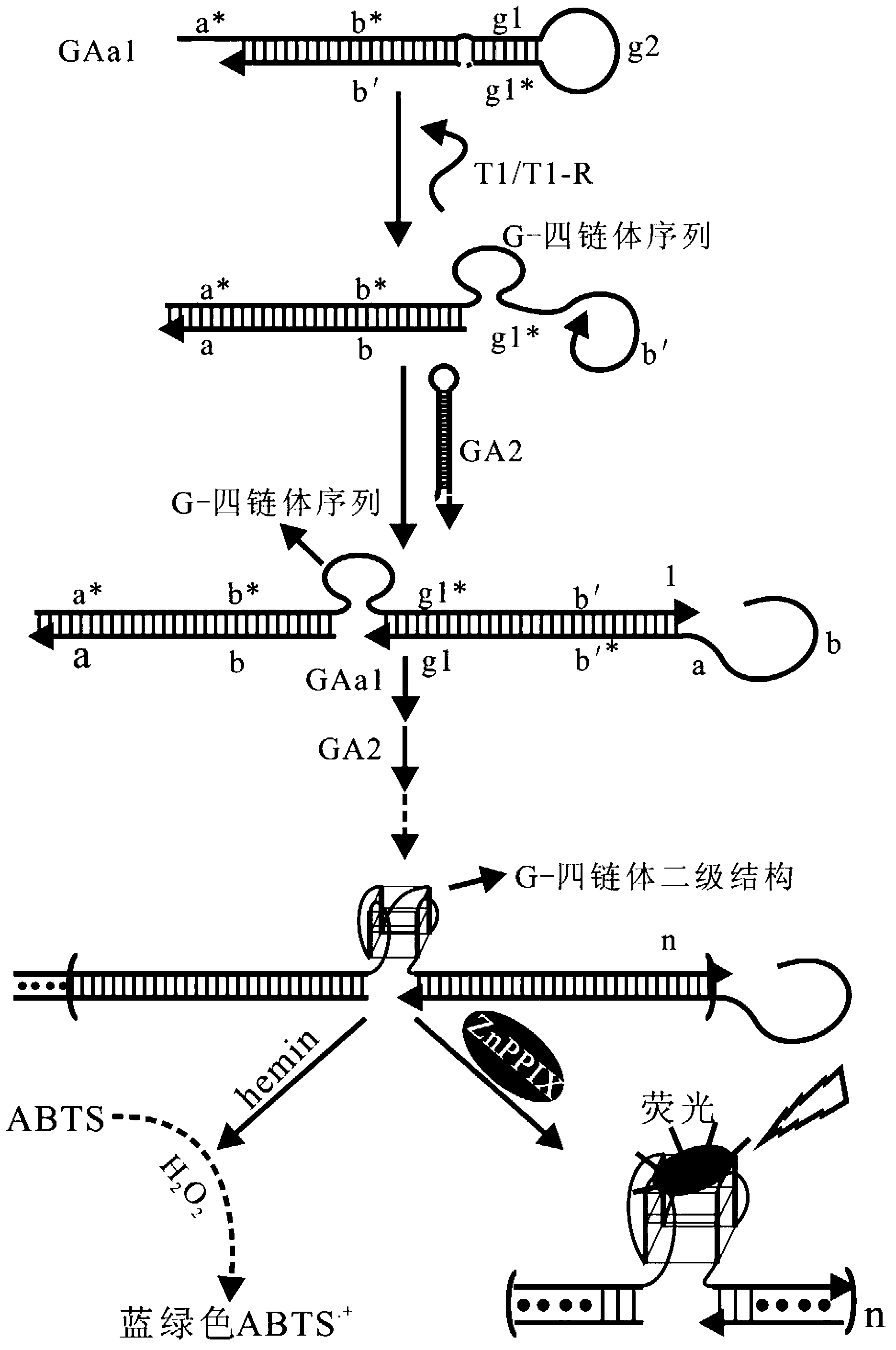
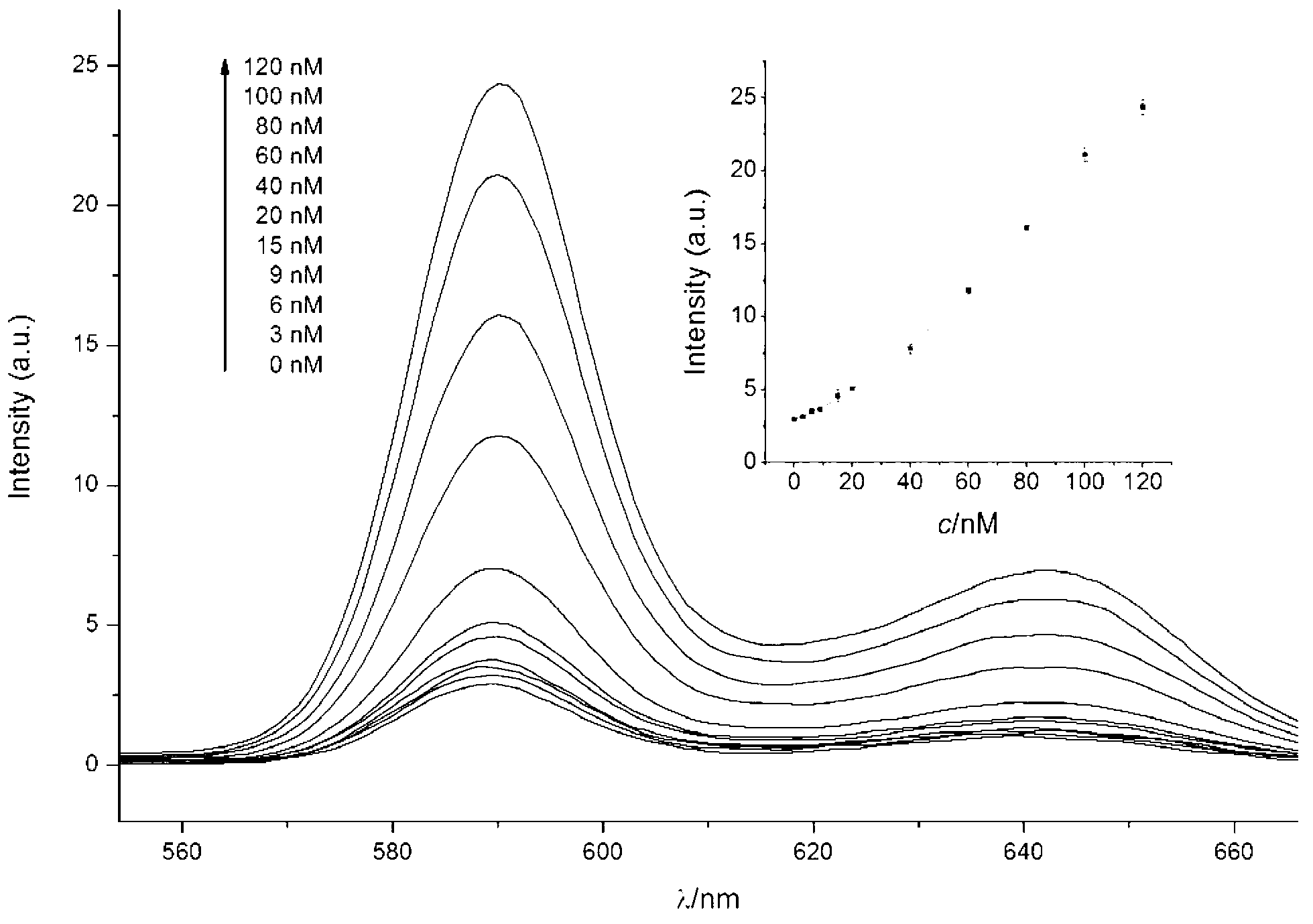
The invention provides a signal amplification and signal reporting integrated oligonucleotide probe. The probe is a pair of DNA hairpin sequences designed on the basis of a target molecule and having cohesive ends, substantially is a product combining a hybridization chain reaction fuel molecule with a G-quadruplex sequence, has the characteristic of target molecule signal cascade amplification of the hybridization chain reaction in an enzyme-free isothermal manner, and enables the amplified target molecule signal to be conveniently and simply detected through the introduction of a signal reporting element G-quadruplex sequence. The invention also provides a method of an enzyme-free isothermal nucleic acid probe mediated by the above probe. The method enables the signal of an object to be detected to be amplified and detected under a normal temperature condition without any enzymes, and has the advantages of sensitivity, accuracy, simplicity, and easy implementation.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
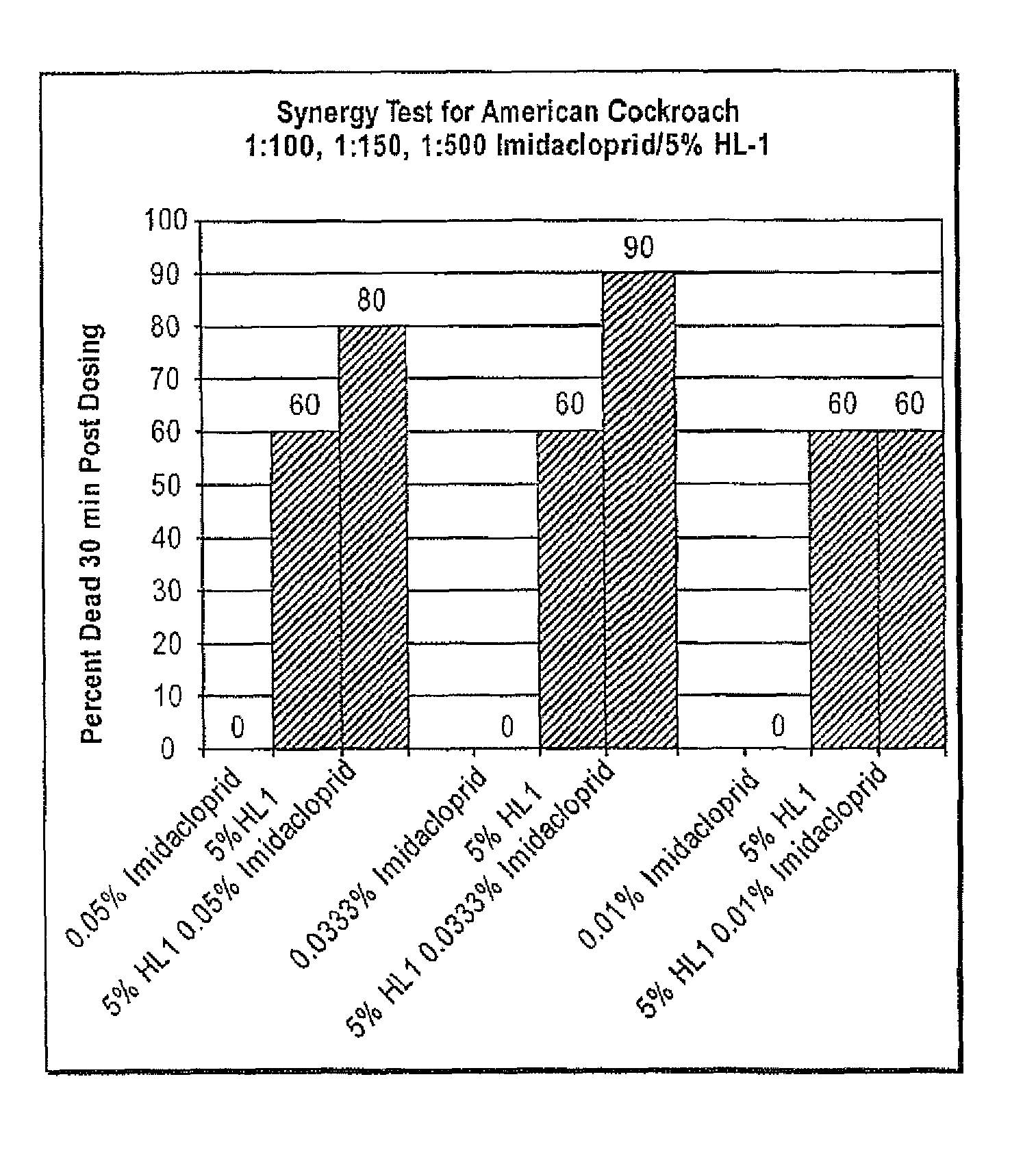
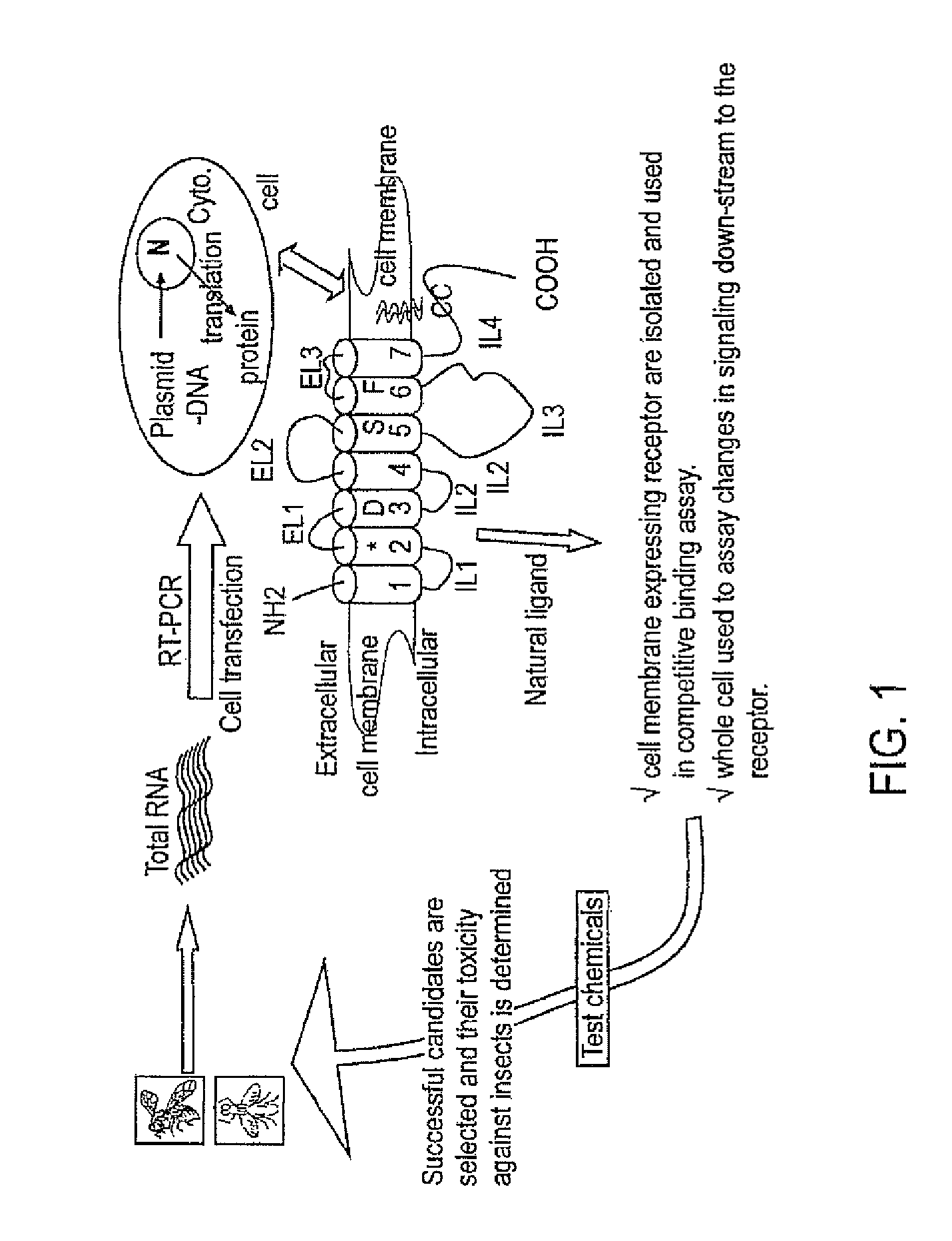
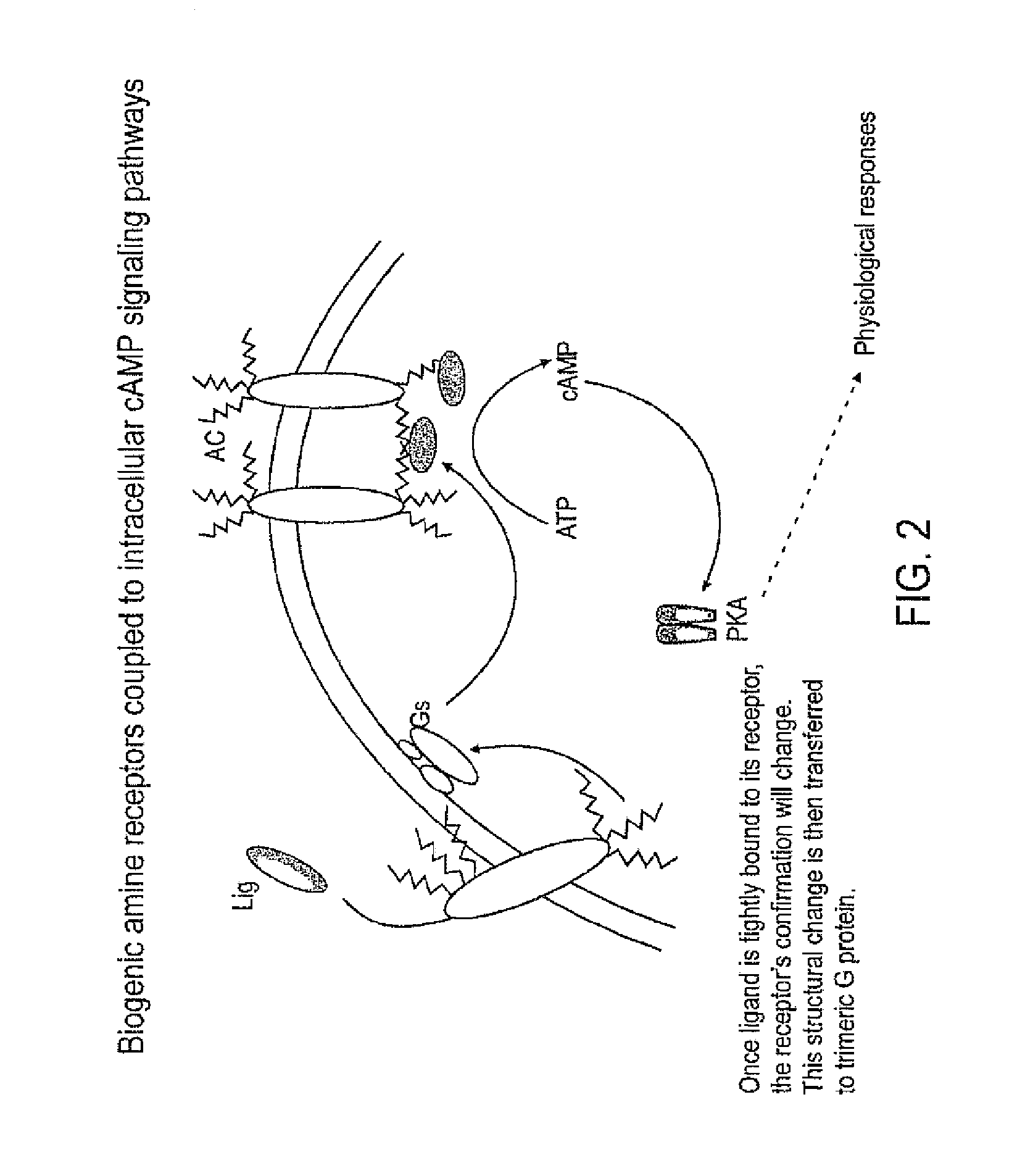
Pest control compositions and methods
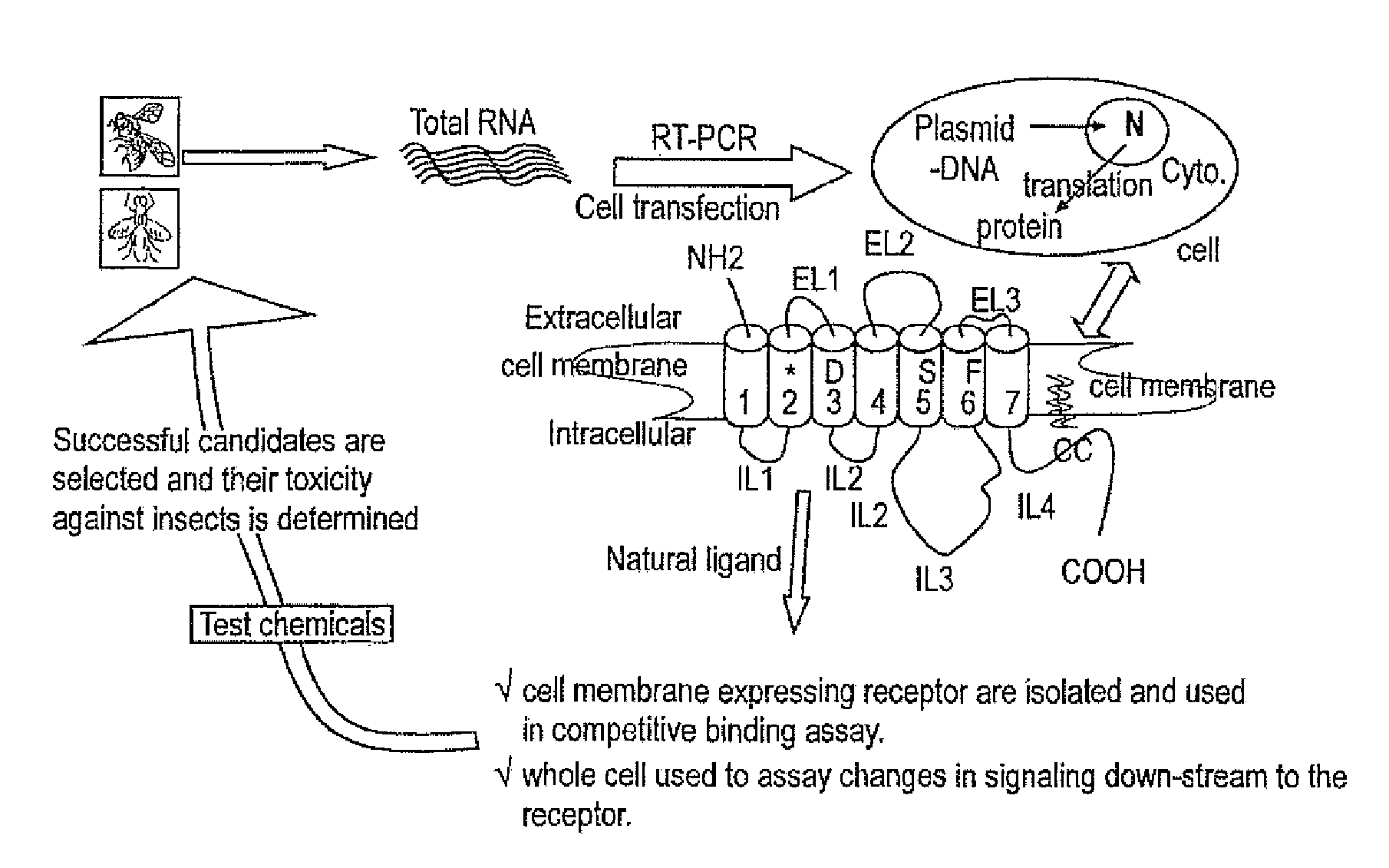
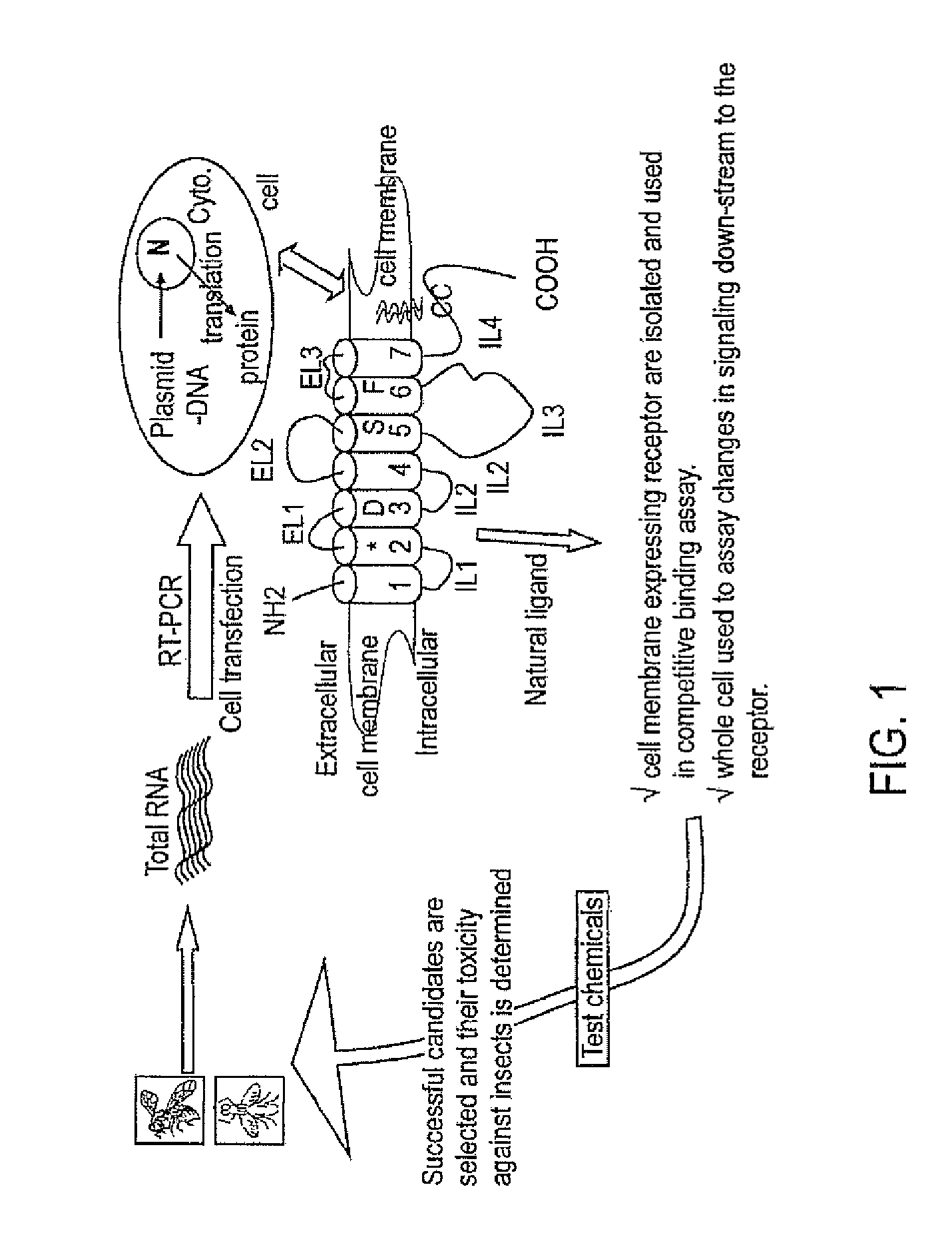
Embodiments of the present invention provide compositions for controlling a target pest including a first agent and a second agent comprising a pest control product or a signal cascade modulator, wherein the first agent and the second agent act synergistically to control the target pest. The first agent can be capable of interacting with a receptor in the target pest. The pest control product can have a first activity against the target pest when applied without the active agent and the compositions can have a second activity against the target pest; and the second activity can be greater than the first activity. Embodiments of the invention can include compositions that modulate the signal cascade initiated by the binding of ligands to, for example, cell surface receptors. Methods of screening such compositions are also disclosed.
Owner:TYRATECH
Use of substances that act as cascade inhibitors of the raf/mek/erk signal cascade, for producing a medicament to treat dna and rna viruses
The invention consists in that substances acting as cascade inhibitors of the Raf / MEK / ERK signaling path-way, in particular MEK inhibitors, are used for the production of a drug for the preventive and antiviral therapy against DNA and RNA viruses, in particular against intranuclear-replicating negative strand RNA viruses, for instance influenza or Borna disease viruses.
Owner:MEDINNOVA FUR MEDIZINISCHE INNOVATIONEN AUS AKADSCHER FORSCHUNG MBH
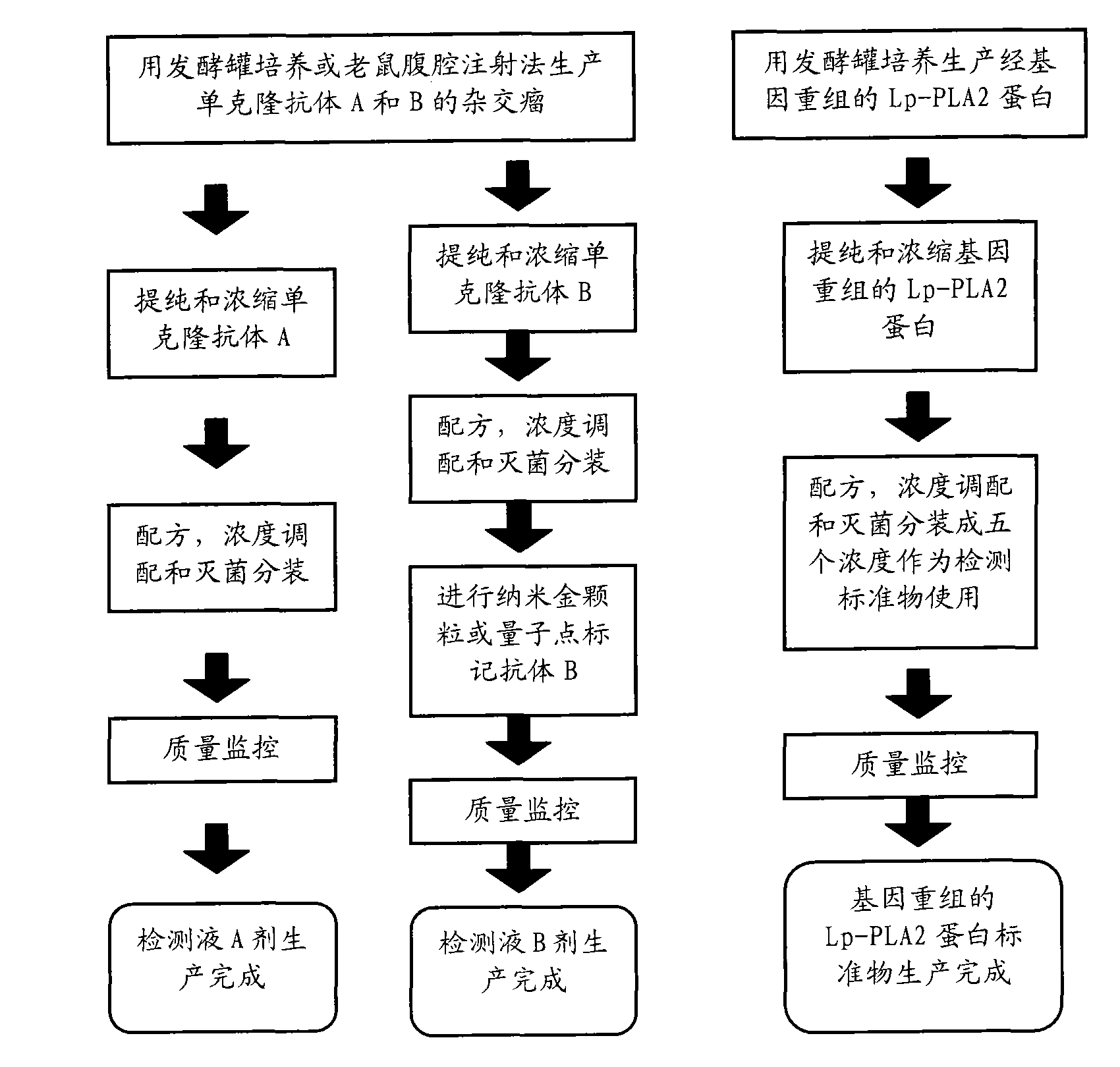
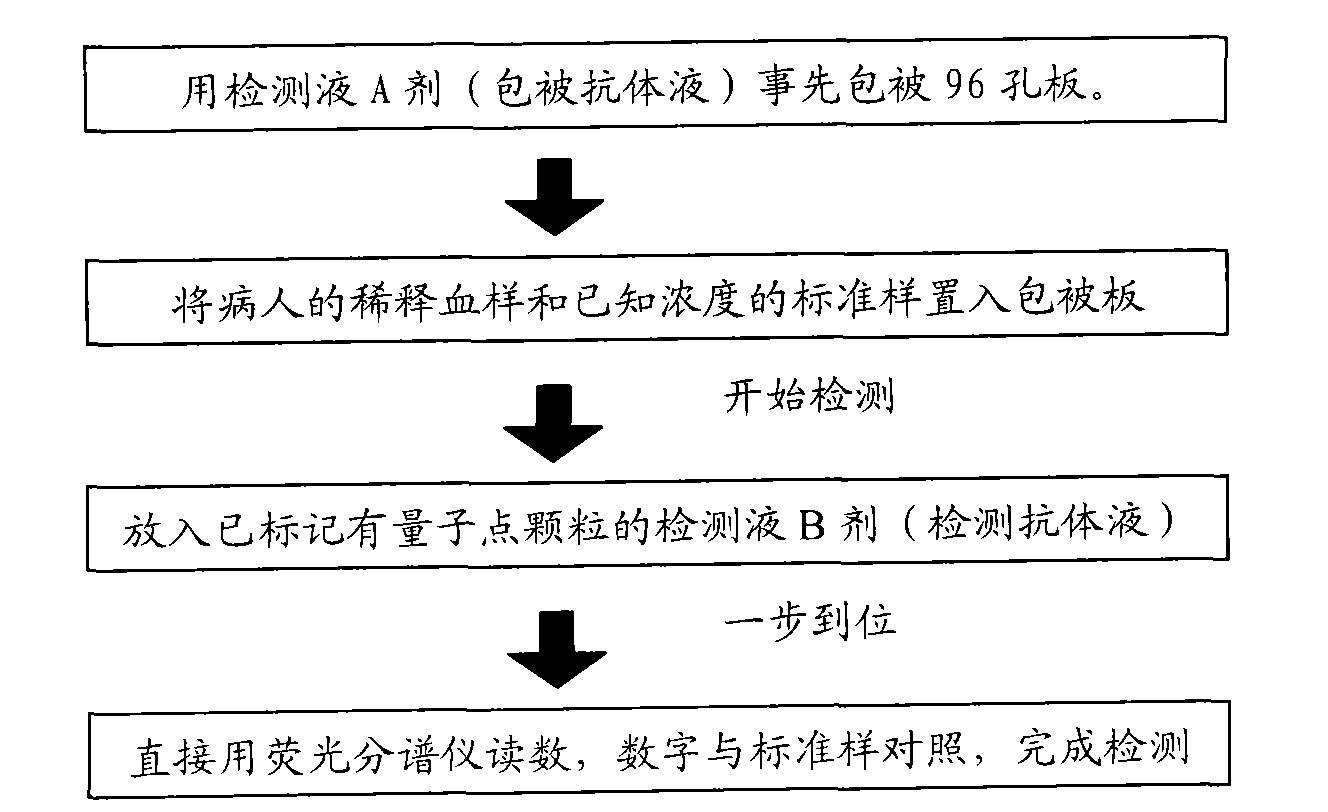
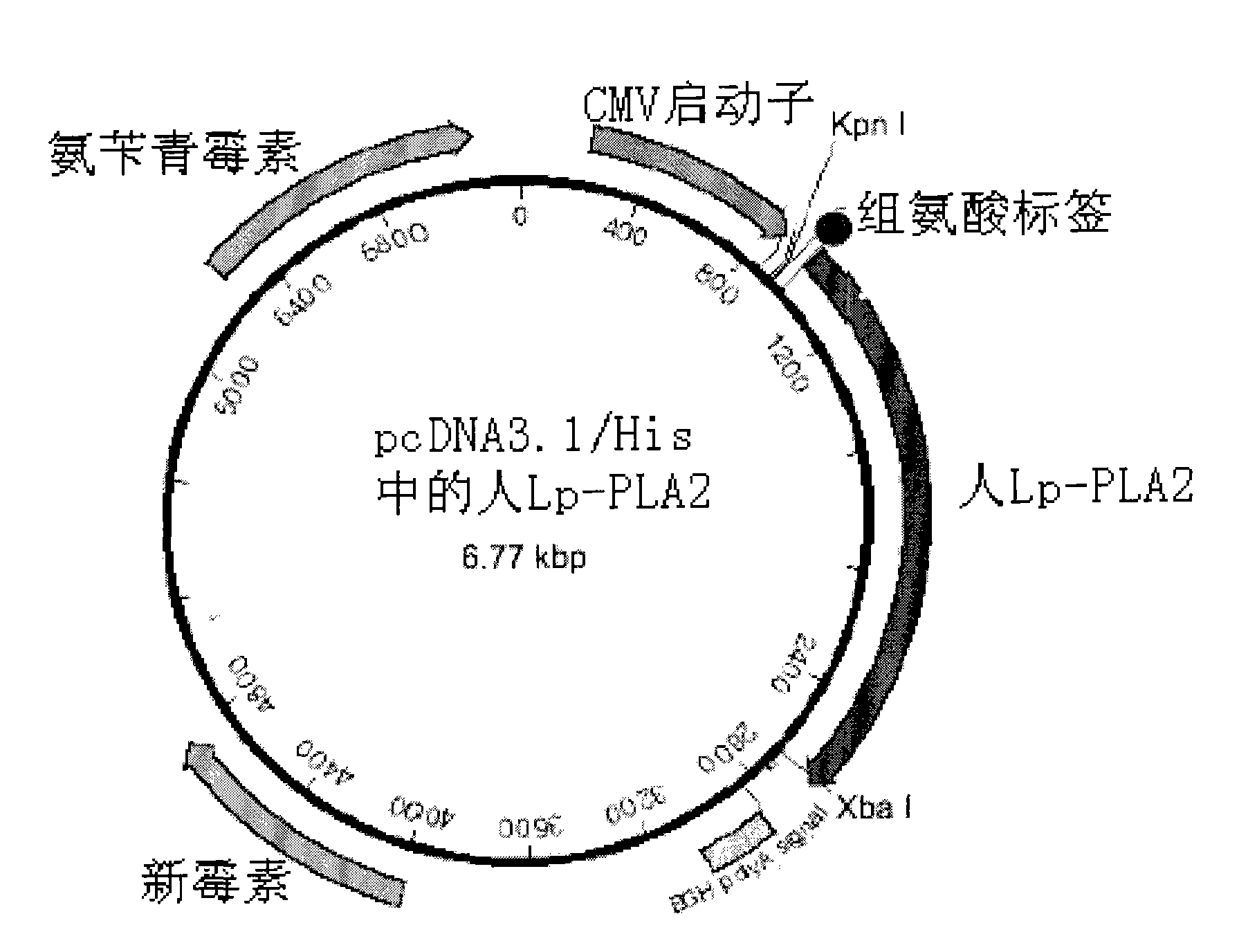
Immunological detecting kit and preparation method and using method thereof
InactiveCN102121938AQuick and time-saving detectionShorten detection timeBiological testingHuman bodyCreatine kinase
The invention provides an immunological detecting kit, a preparation method and a using method thereof. The kit comprises a protein antibody which is to be detected and is marked by a marker capable of directly detecting, wherein the protein to be detected is the protein related to heart cerebrovascular disease examination. The protein to be detected comprises one or more of fibrinogen, C-reactive protein, thrombus precussor protein, creatine kinase and human body lipoprotein related phospholipase A2. The abundance or the concentration of the protein to be detected in the sample to be detected can be detected in one step by using the kit, so the step and the time are saved; and compared with the traditional method requiring signal cascade amplification such as enzyme-linked immuno sorbent assay (ELISA) and the like, the accuracy is improved.
Owner:TIANJIN KANGERKE BIOSCI
Pest control compositions and methods
Embodiments of the present invention provide compositions for controlling a target pest including a first agent and a second agent comprising a pest control product or a signal cascade modulator, wherein the first agent and the second agent act synergistically to control the target pest. The first agent can be capable of interacting with a receptor in the target pest. The pest control product can have a first activity against the target pest when applied without the active agent and the compositions can have a second activity against the target pest; and the second activity can be greater than the first activity. Embodiments of the invention can include compositions that modulate the signal cascade initiated by the binding of ligands to, for example, cell surface receptors. Methods of screening such compositions are also disclosed.
Owner:TYRATECH
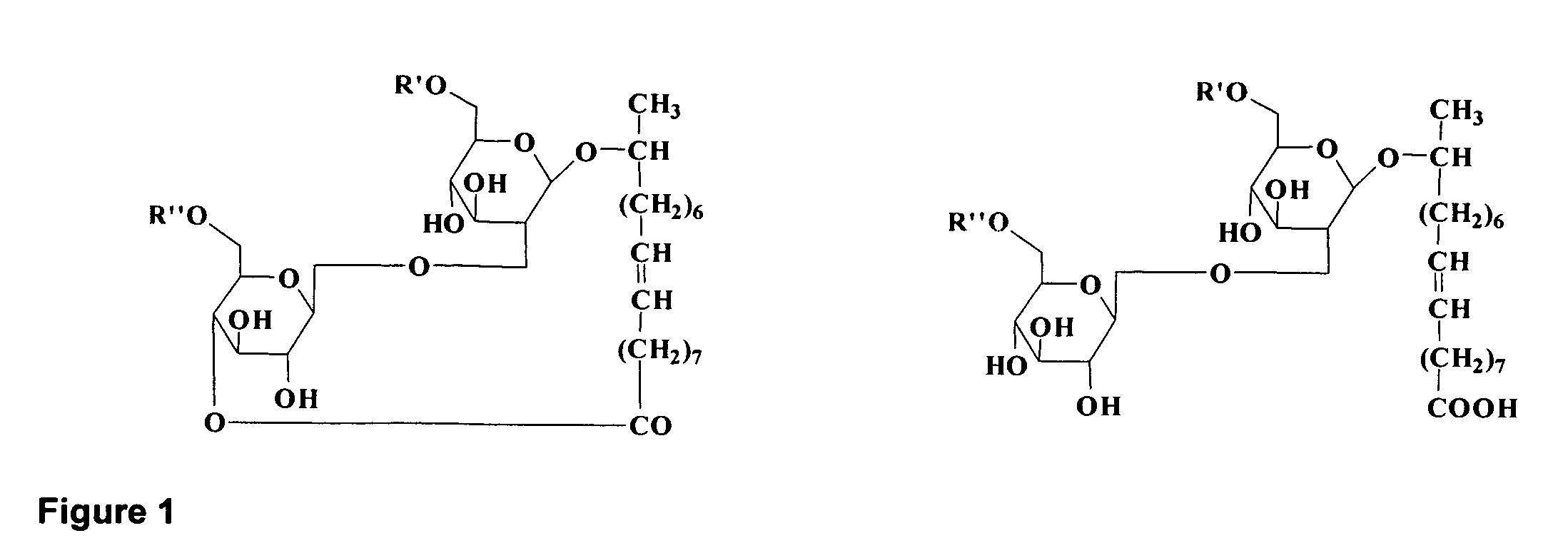
Purified Ethyl Ester Sophorolipid for the Treatment of Sepsis
InactiveUS20120142621A1High purityEfficient productionBiocideCarbohydrate active ingredients[Candida] apicolaChemical transformation
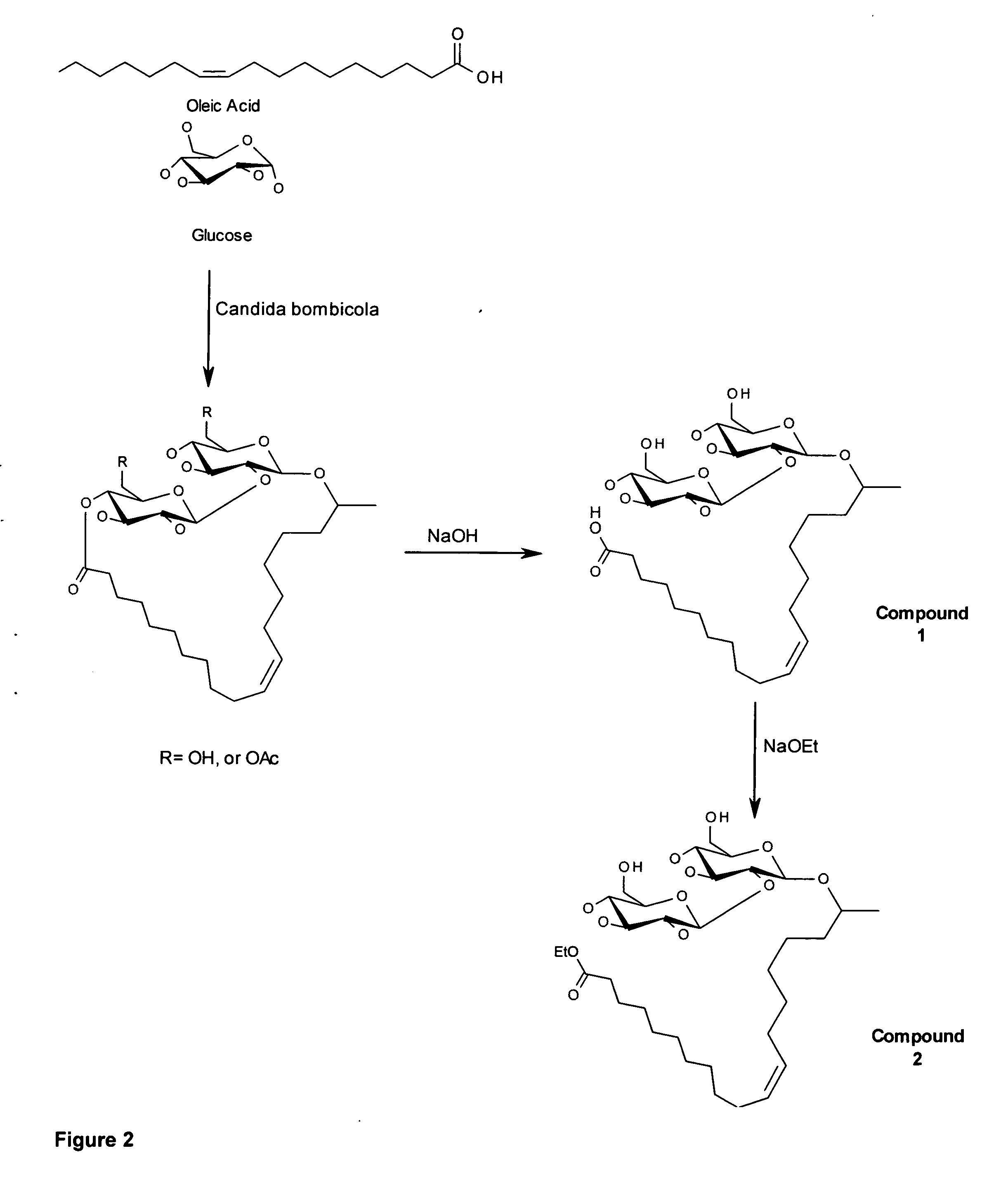
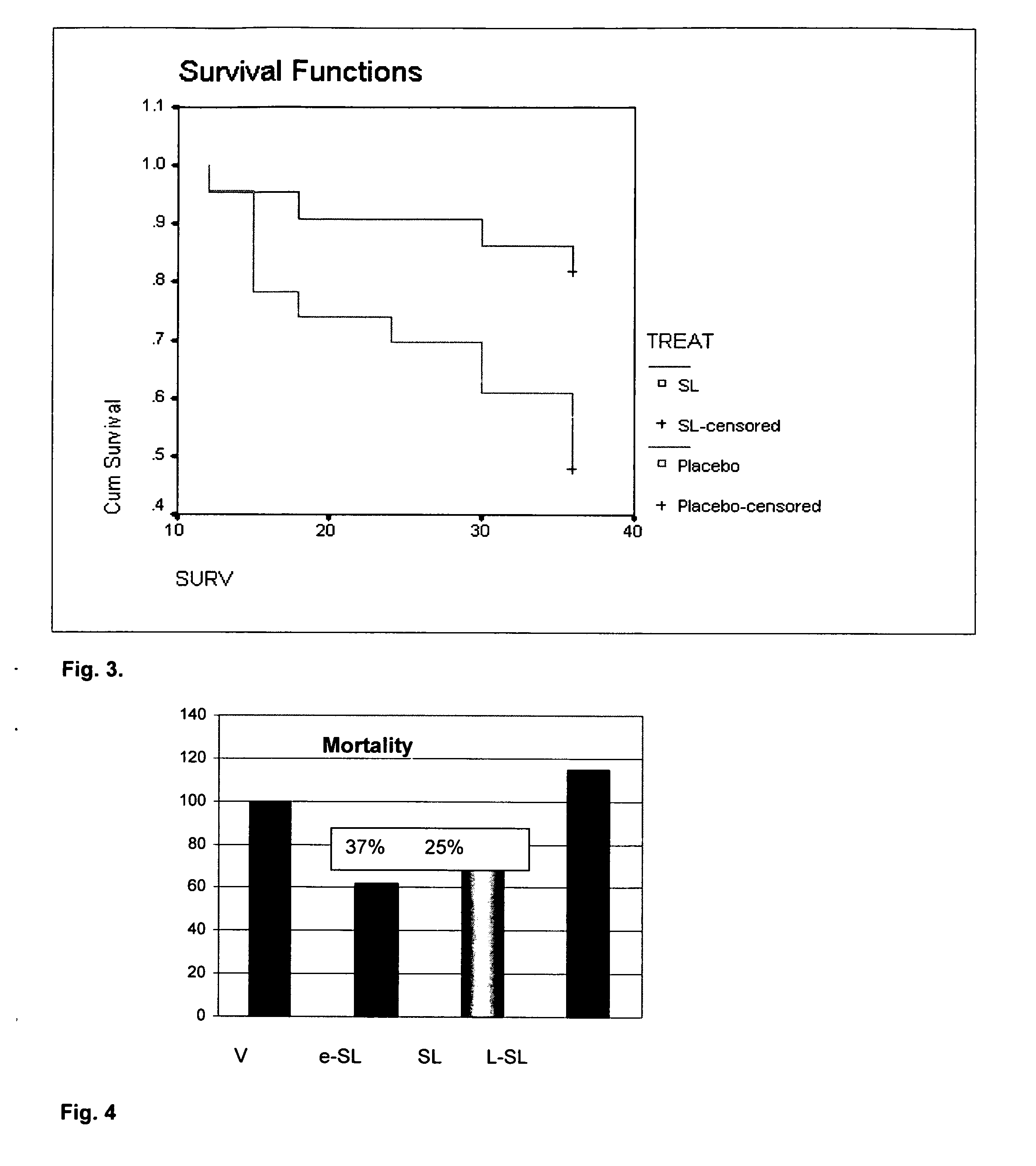
A microbial ethyl esther sophorolipid derivative with no acetylated groups produced by Candida species, for treating and preventing sepsis / septic shock. The method of producing sophorolipids is through microbial resting cells of Candida bombicola. The sophorolipids obtained from resting state cultures are isolated as a complex mixture of compounds and then decanted as a dense oil from the culture broth, subsequently washed to remove free fatty acids. Secondary chemical transformation via base catalyzed hydrolysis is used to reduce the 8 possible structural sophorolipid species to a single moiety, the 17-L-[(2′-O-b-D-glucopyranosyl-b-D-glucopyranosyl)-oxy]-cis-9-octadecenoate de-acetylated free acid. The compound acts primarily through decreasing inflammatory cytokines and eliciting other synergistic anti-inflammatory mechanisms by blocking TLR4-CD14 upstream of the inflammatory signaling cascade. The compound can be administered either intraperitoneally or intravenously at single or multiple doses of 5-30 mg / kg of weight in solvent media or in capped nanoparticles, preferably within 48 hours of sepsis inception.
Owner:STREETCAREC SLOAN
Method of colorimetric detection of target DNA by combining nanometer gold with polythiophene ramification
InactiveCN101818198AEasy to detectEasy to operateMicrobiological testing/measurementColor/spectral properties measurementsPolythiophene derivativeThiophene derivatives
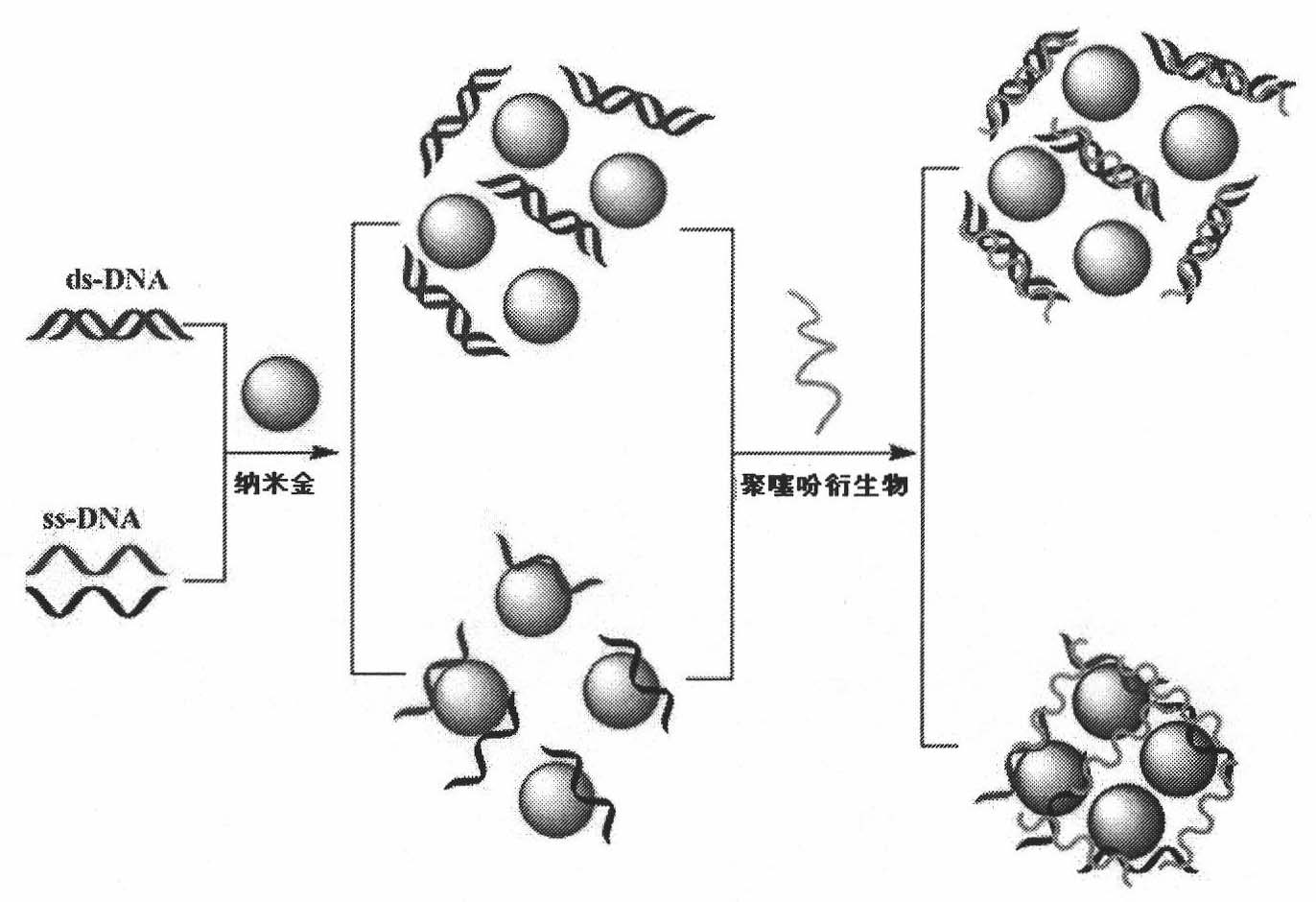
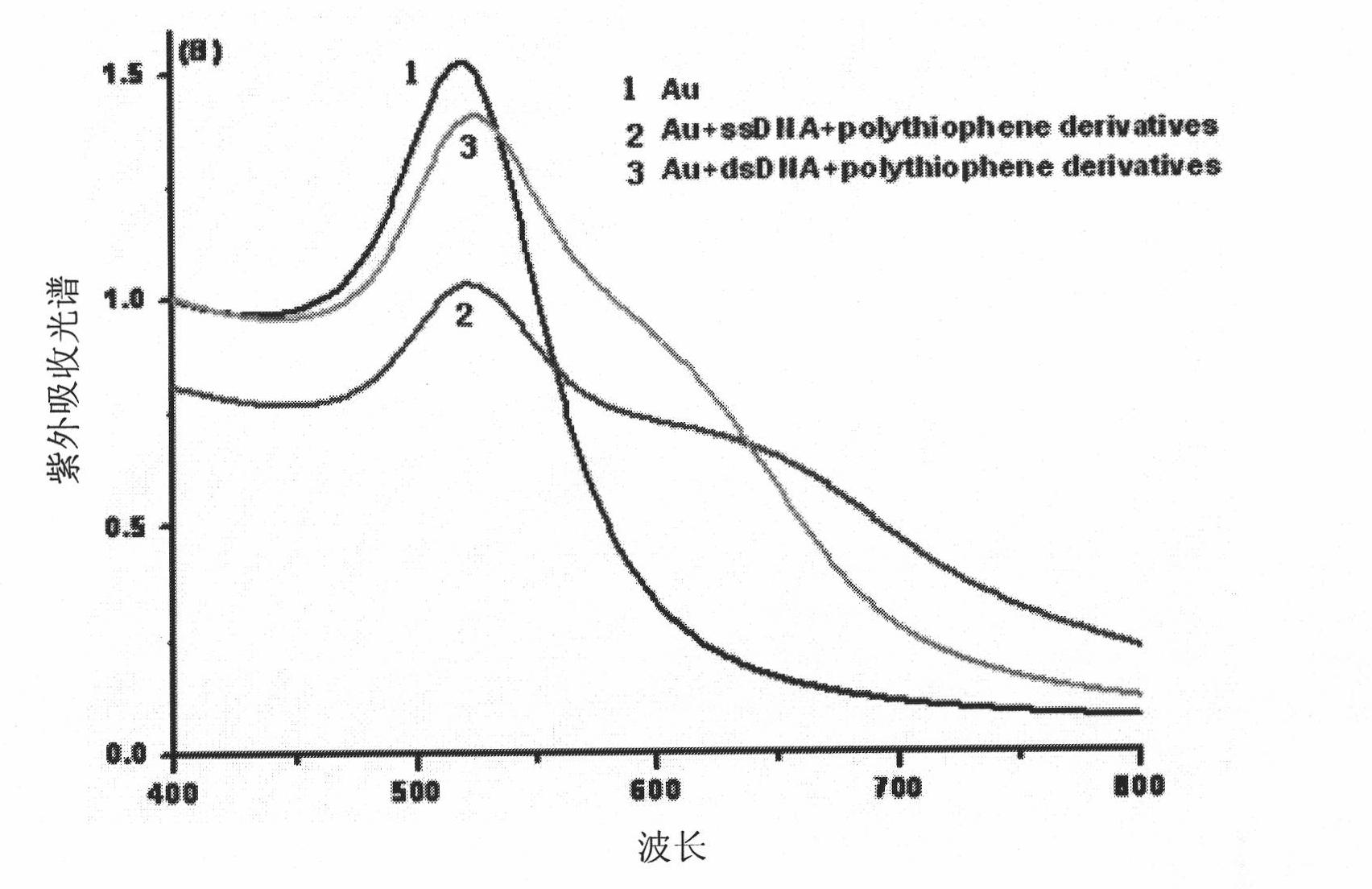
The invention relates to a method of the colorimetric detection of target DNA by combining nanometer gold with polythiophene ramification, which comprises the following steps of: preparing a detection reagent (the nanometer gold and the polythiophene ramification) and a probe; and detecting the sequence of the target, etc. Based on the principle that a signal cascade is amplified due to the change of the color since the stability of the NDA-nanometer golden solution is changed when the polythiophene ramification is combined with the ssDNA / dDNA, the method realizes the high-sensitivity and fast detection of the DNA under the double action of a color converting developer and an amplified label, thereby building an analysis platform which directly detects the gene by combining the nanometer gold with the polythiophene ramification. The method has simple operation, does not need special instrument and equipment, has the characteristics of specificity, speediness and high sensitivity, and can be applied to the diagnosis and the detection of the target DNA in the clinical laboratory medicine and the environment.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Method for determining receptor affinity of GLP-1 receptor agonist
InactiveCN104846061AQuick evaluationQuick filterMicrobiological testing/measurementCytosolResponse element
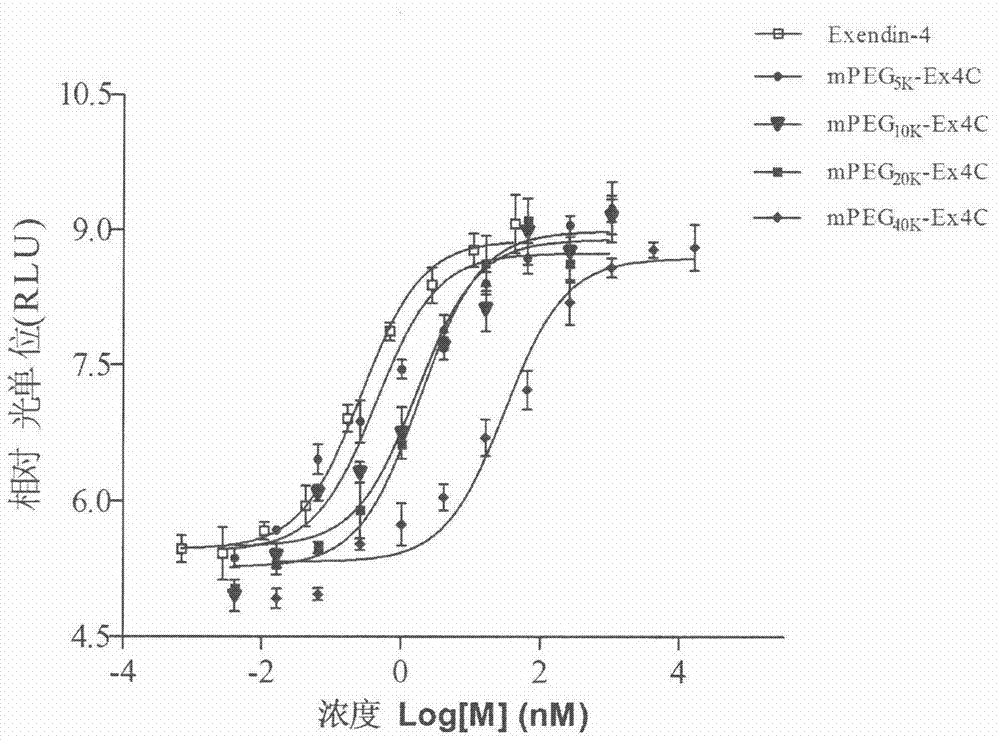
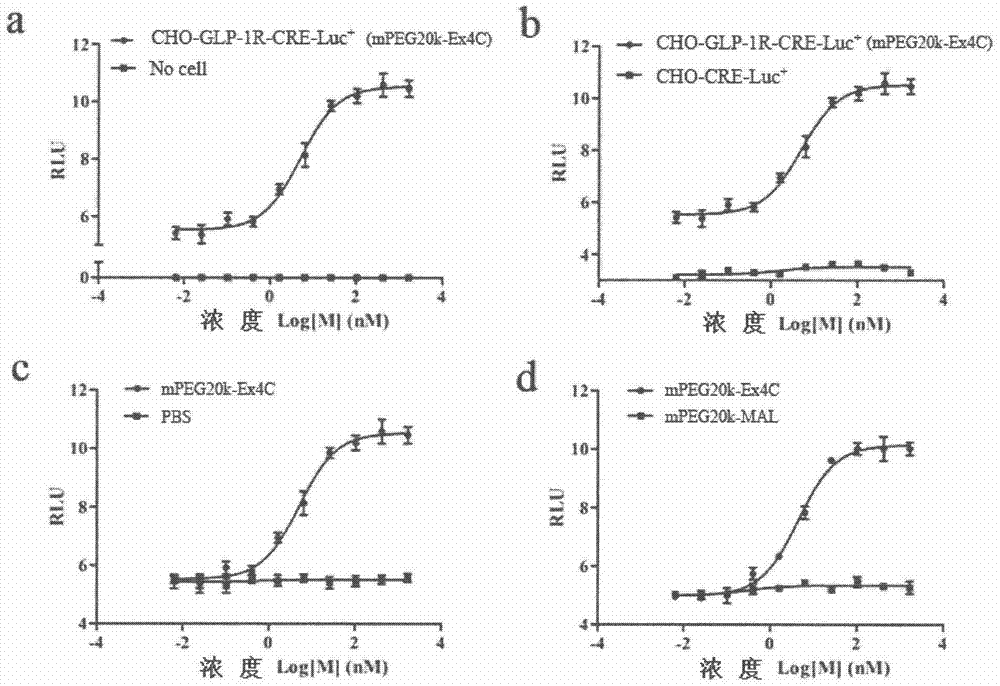
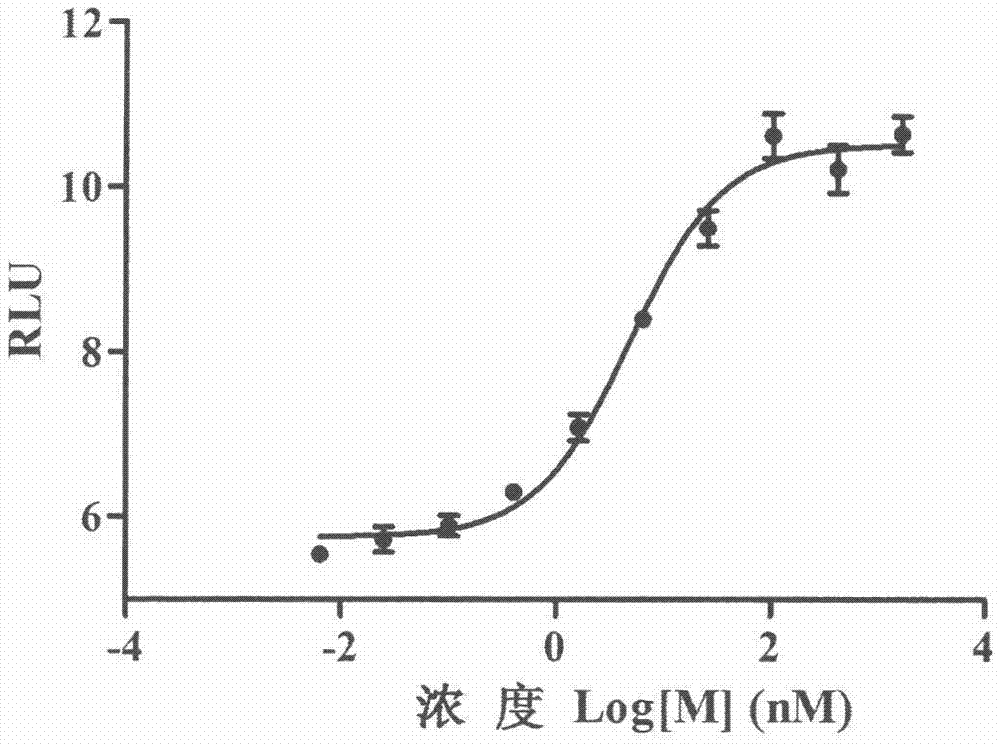
The invention provides a method for determining the receptor affinity of a GLP-1 receptor agonist. The method is based on a tool cell line, namely CHO-GLP-1R-CRE-Luc<+>, when a sample to be determined is combined with GLP-1 receptor on the surface of a cell, the receptor-mediated signal cascade reaction can be activated, cAMP-response element (CRE) is activated specifically, the expression of the luciferase reporter gene is promoted, the quantity of luciferase in cytosol is detected for drawing and fitting to obtain a dose-effect curve of acting of the sample and the GLP-1 receptor; and the half effective concentration (EC50) is calculated. By inspecting and optimizing all influence factors in the determining process, the method for determining the receptor affinity of the GLP-1 receptor agonist is finally established. The method has the advantages of being high in specificity, precision and accuracy, good in durability and convenient to operate, and the like. The method can be used for determining the receptor affinity of the GLP-1 receptor agonist, and thus such type of drug can be fast evaluated and screened.
Owner:CHINA PHARM UNIV
Micro-RNA-21 ultra-sensitive detection method based on double-enzyme signal cascade amplification
InactiveCN108034697AEnables ultra-sensitive detectionHigh sensitivityMicrobiological testing/measurementEthylene DichlorideColor changes
The invention discloses a micro-RNA-21 ultra-sensitive detection method based on double-enzyme signal cascade amplification. The method includes the steps: horseradish peroxidase enzyme-labeled probepreparation: coupling carboxylation polystyrene micro-spheres and horseradish peroxidase enzyme through DNA (deoxyribonucleic acid) single chains by an EDC (ethylene dichloride) carboxyl and amino coupling method; double-chain specific nuclease assisted target recycling: specifically cutting the DNA single chains complementarily paired with RNA (ribonucleic acid) to be detected in a probe by double-chain specific nuclease, triggering target circular reaction, releasing more horseradish peroxidase enzyme by cutting and amplifying signals; horseradish peroxidase enzyme signal amplification: collecting the horseradish peroxidase enzyme released in supernatant by cutting, adding a TMB (tetramethylbenzidine) chromogenic substrate, realizing naked-eye qualitative diagnosis by color change, and realizing enzyme-labeled quantification according to change of absorbance values. The difference of micro-RNA family members is effectively distinguished, and the method is suitable for community tumorscreening of high risk groups and has great application values in biomedical research and clinical diagnosis.
Owner:TIANJIN UNIV
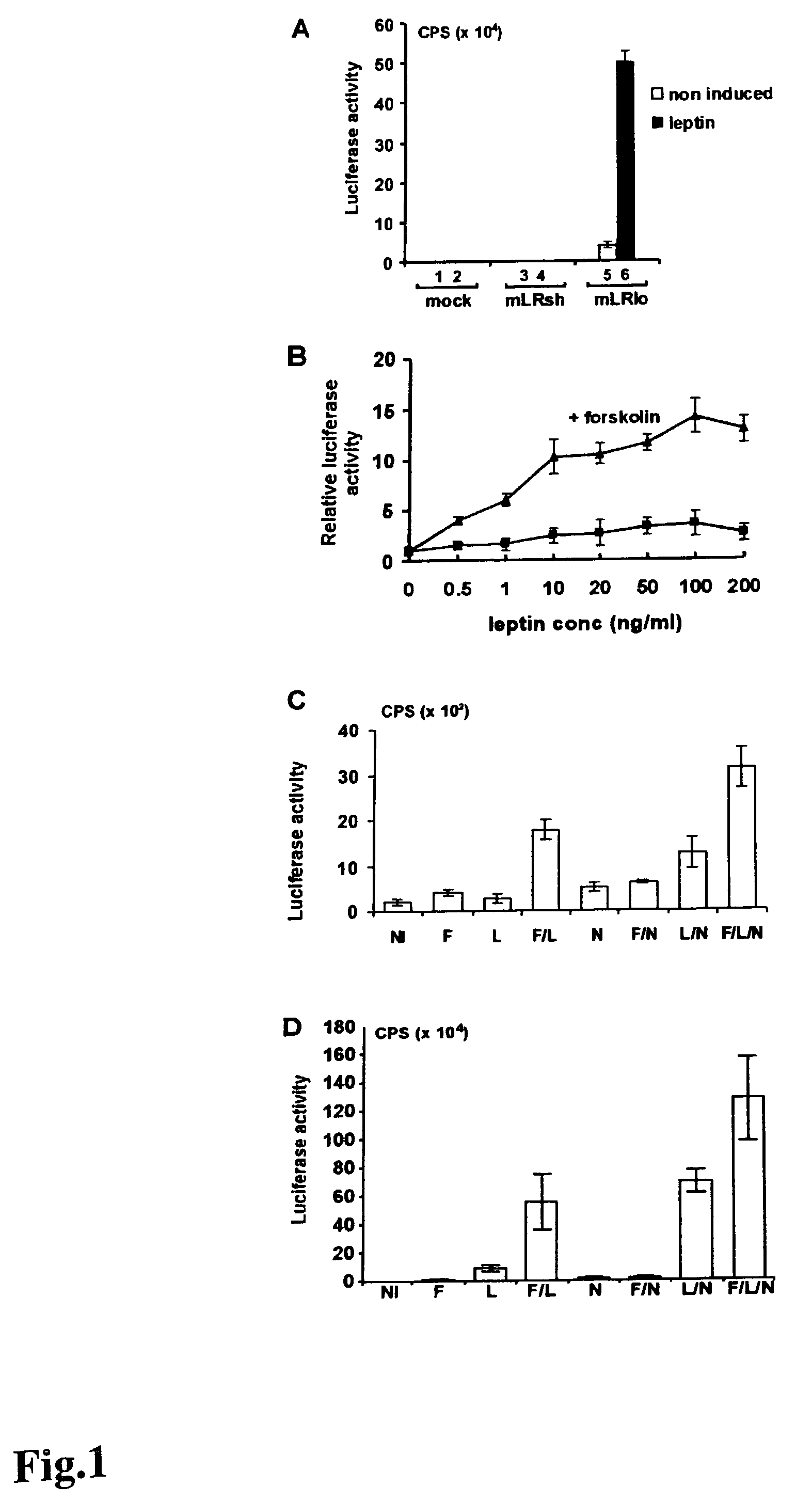
Leptin-mediated gene-induction
InactiveUS7291458B2Inducing effectObesity gene productsPeptide/protein ingredientsProtein iThreonine
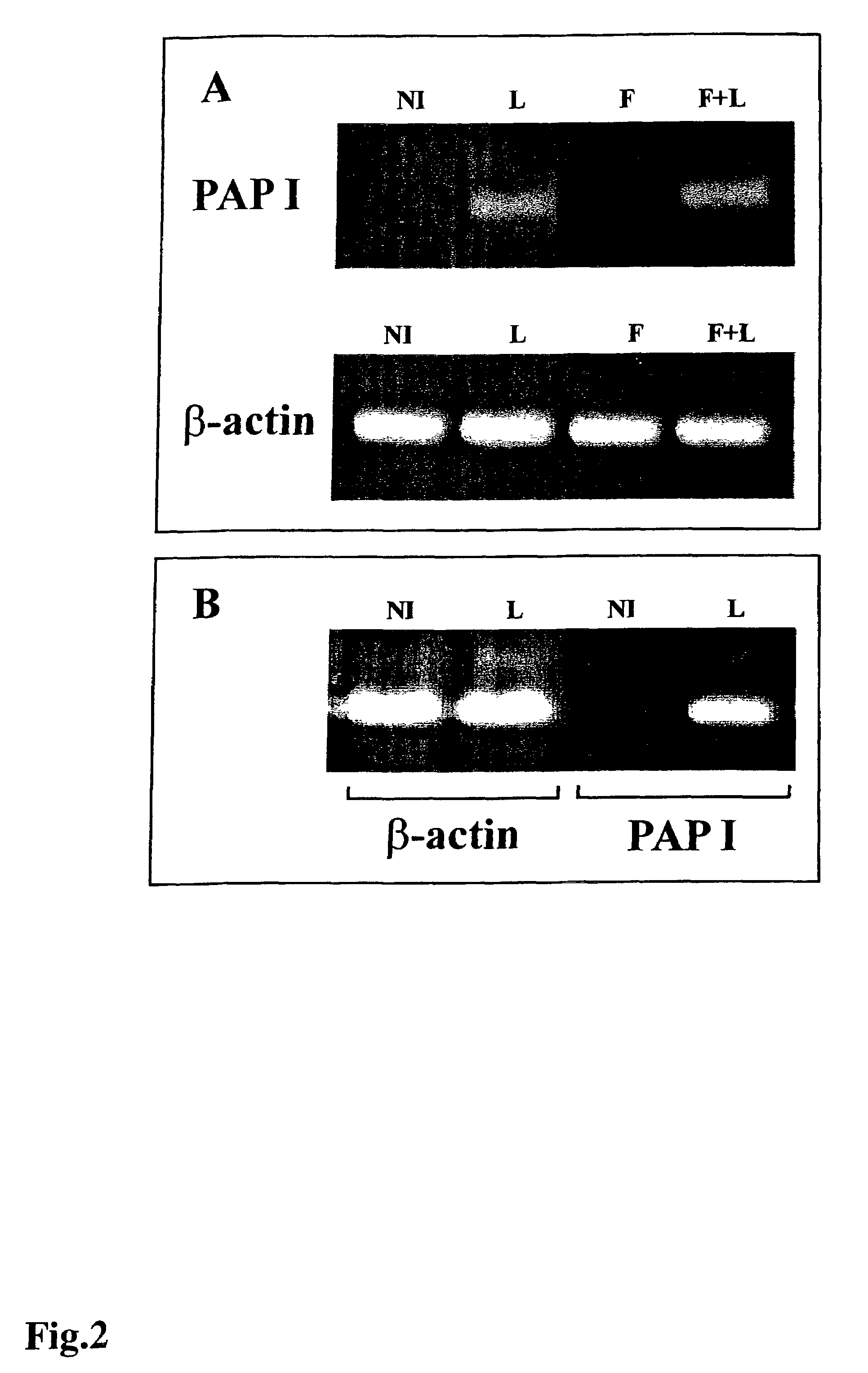
Methods of activating a signaling cascade comprising, introducing leptin and / or a cytokine to a receptor complex comprising gp 130, optionally in combination with a compound acting on adenylate cyclase or acting on one or more downstream targets of adenylate cyclase, thereby inducing genes in neuro-endocrine cells or cells of neuro-endocrine origin. Two distinct gene-sets are induced, immediate early response genes (STAT-3, SOCS-3, Metallothionein-II, the serine / threonine kinase Fnk and the rat homologue of MRF-1), and late induced target genes (Pancreatitis Associated Protein I, Squalene Epoxidase, Uridinediphosphate Glucuronyl Transferase and Annexin VIII). Strong co-stimulation with the adenylate cyclase activator forskolin was shown with respect to late induced target genes. Transcripts encoding Leptin Induced Protein I (LIP-I) and Leptin Induced Protein II (LIP-II) were identified; however, no forskolin co-stimulatory effect was observed. It is also demonstrated that leptin modulates in vivo expression of MT-II, Fnk and Pancreatitis Associated Protein I genes.
Owner:NEC CORP +1
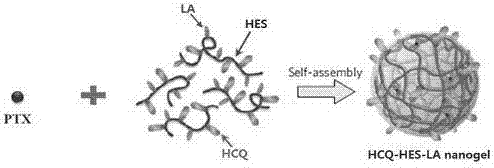
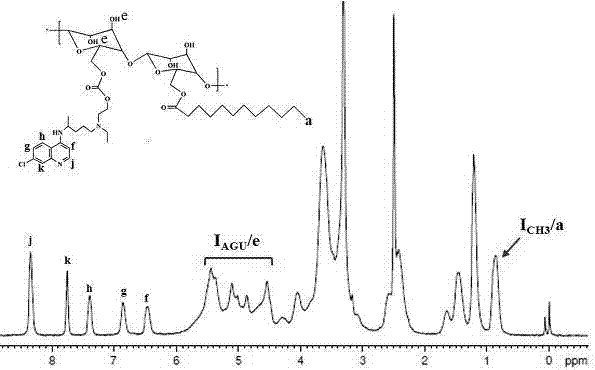
Chloroquine-polymerized nanogel delivery system and preparation method thereof
InactiveCN107375199APrevent proliferationStable structureAerosol deliveryOintment deliveryGene deliveryLymphatic Spread
The invention discloses a chloroquine-polymerized nanogel delivery system and a preparation method thereof. The delivery system contains chloroquine-polymerized nanogel particles, and antitumor medicines which are wrapped with the particles, wherein the chloroquine-polymerized nanogel is prepared through hydroxychloroquine and polysaccharide framework which is modified through a hydrophobic side chain. According to the medicine delivery system, malignant tumor and tumor metastasis are treated synchronously; the signal cascade of cancer cells metastasizing to surrounding and distant sides is stopped while cancer cell proliferation is inhibited, and malignant cancer can be effectively treated.
Owner:CHINA PHARM UNIV
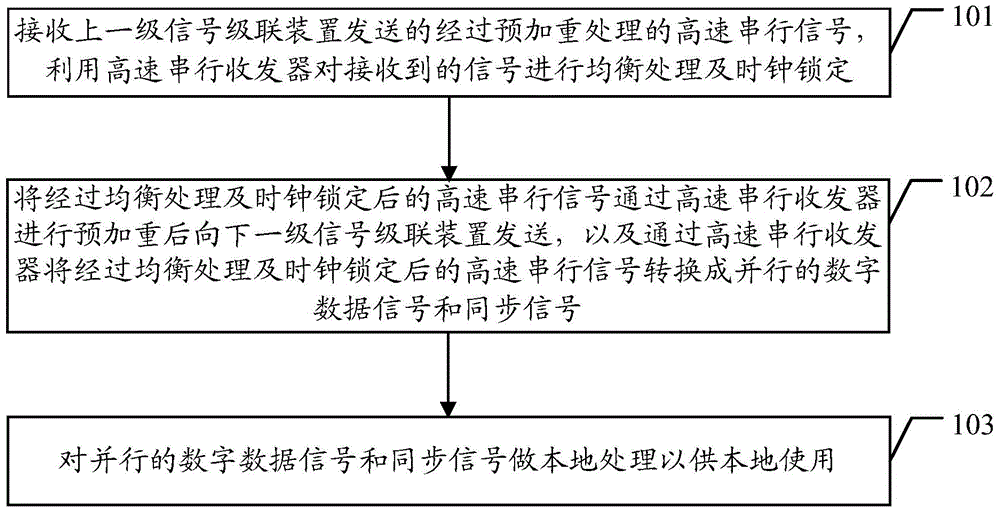
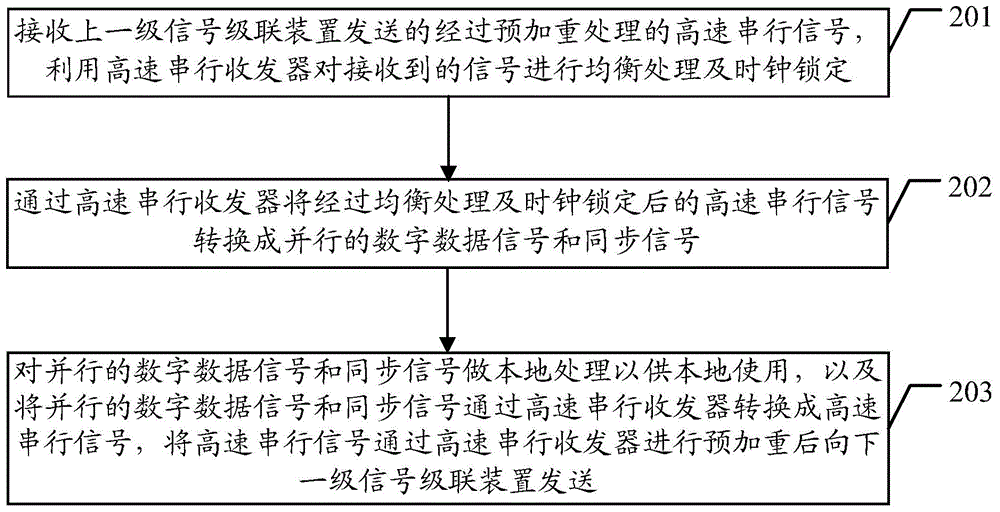
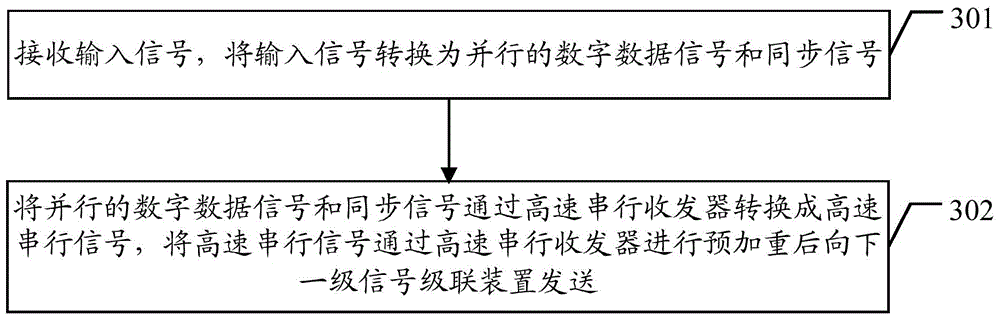
Signal cascade transmission method and signal cascade device
ActiveCN103606367ARealize cascadingQuality improvementCathode-ray tube indicatorsDigital dataHigh bandwidth
The embodiment of the invention discloses a signal cascade transmission method and a signal cascade device. The method includes the following steps that a high-speed serial signal which is sent by a former-series signal cascade device and has been subject to pre-emphasis processing is received and is subject to equilibrium processing and clock locking through a high-speed serial sending and receiving device; the high-speed serial signal which has been subject to equilibrium processing and clock locking is sent to a next-series signal cascade device after being subject to pre-emphasis processing through the high-speed serial sending and receiving device, and the high-speed serial signal which has been subject to equilibrium processing and clock locking is converted into a digital data signal and a synchronizing signal which are parallel through the high-speed serial sending and receiving device; the digital data signal and the synchronizing signal which are parallel are subject to local processing so as to be locally used. The signal cascade transmission method and the signal cascade device can solve the problem that cascade series are limited during signal cascade in the prior art, and cascade of high-bandwidth data can be achieved.
Owner:GUANGDONG VTRON TECH CO LTD
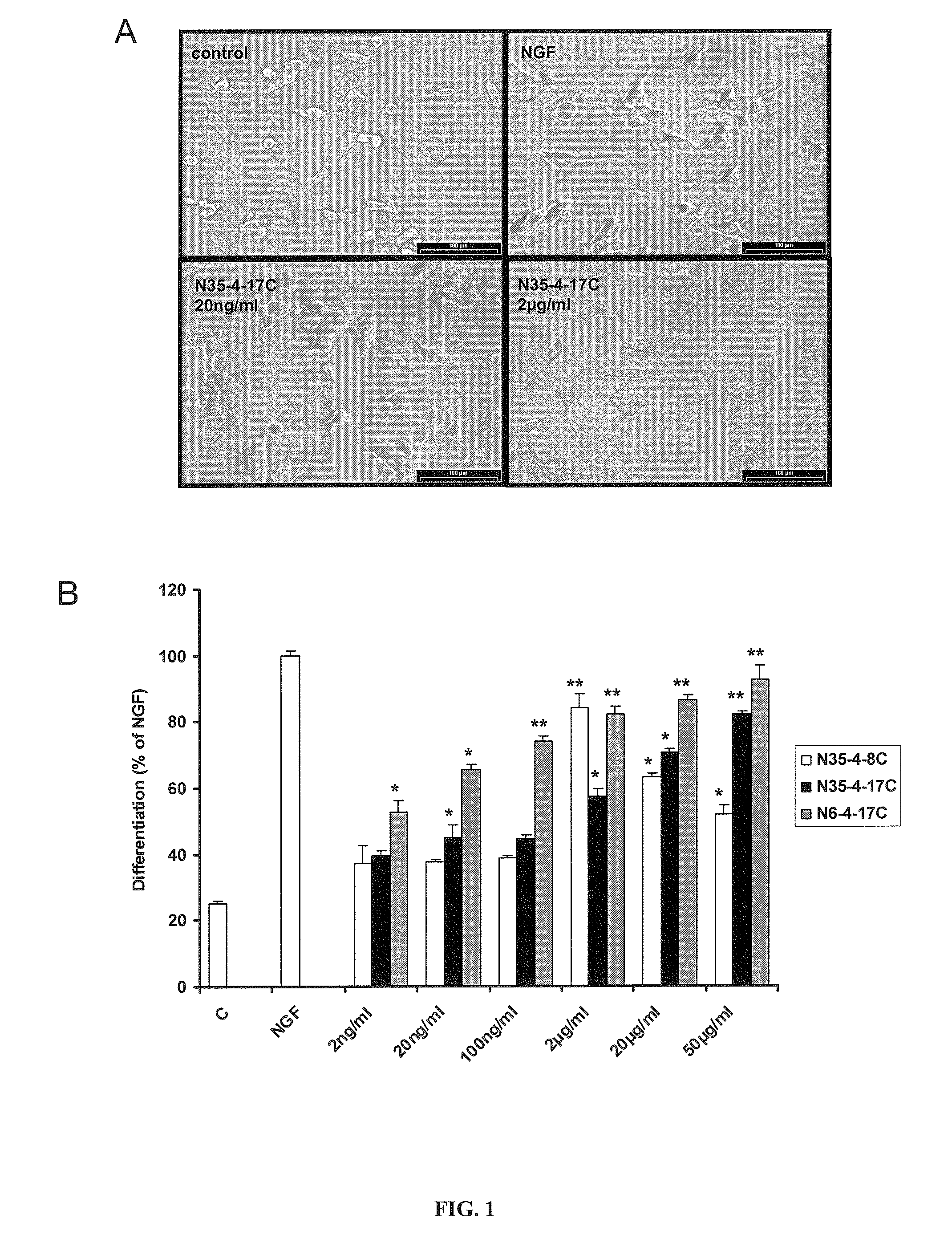
Peptoid Agonists of Nerve Growth Factor and Their Use as Medicaments
InactiveUS20120237552A1Preventing and treating nerve cell deathPreventing and treating and damageSenses disorderNervous disorderSide effectHalf-life
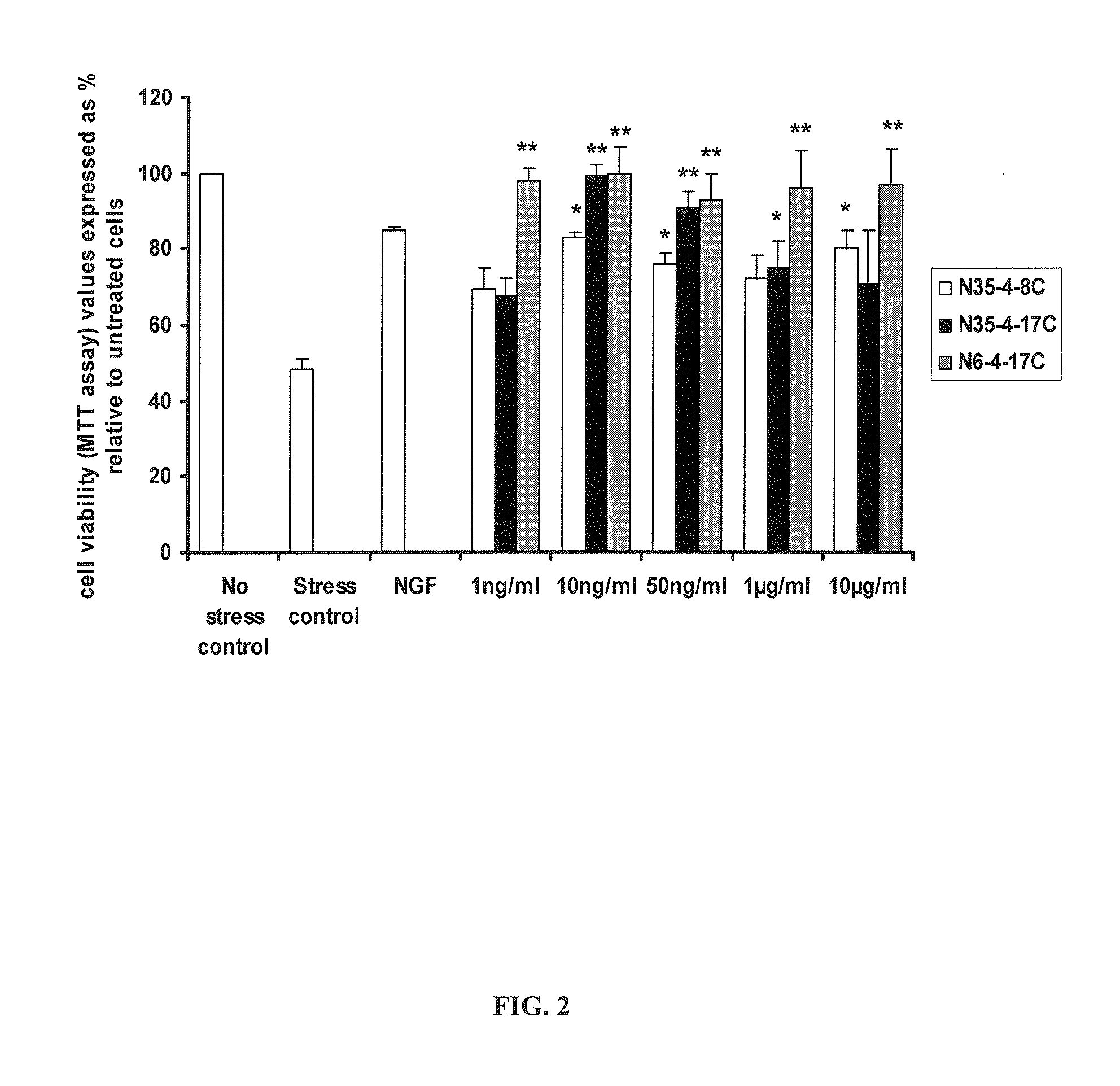
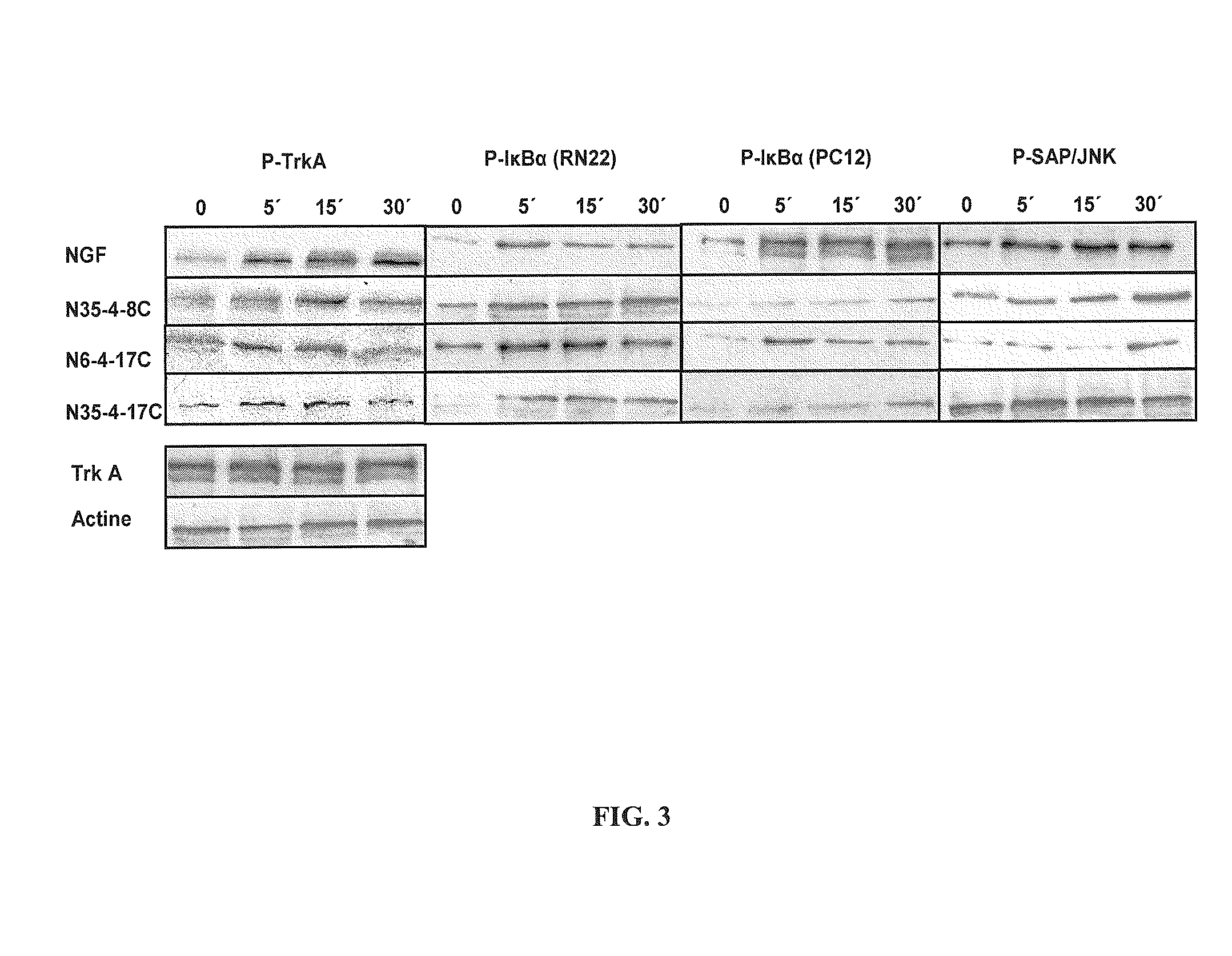
Neurotrophin binding to its specific receptors Trk A and p75 leads to the activation of multiple signalling cascades, culminating in neuroprotective and regenerative effects, including neuronal survival and neurite outgrowth. Neurotrophic factors have been used for the treatment of several neurodegenerative diseases. However, their use is limited by their inability to cross the blood-brain barrier, their short half life and their side effects. Small molecule neurotrophin mimetics may be beneficial in treating a number of neurodegenerative disorders. The present invention shows the capacity of nerve growth factor agonist molecules of Formulae I-IV, as defined in the specification, to induce differentiation in PC 12 cells, promote survival in RN22 cells and activate Trk A, IkBa and SAPK / JNK phosphorylation to various extents in both cell lines. In addition these molecules were able to ameliorate acute experimental autoimmune encephalomyelitis (EAE), a multiple sclerosis (MS) animal model, inhibiting brain inflammation and reducing brain damage. We also observed suppression in the production of pro-inflammatory genes like the inducible nitric oxide synthase. These small molecules with NGF agonist activity may be beneficial for MS and other neurodegenerative diseases due to its neuroprotective and immunomodulatory properties.
Owner:MORENO BEATRIZ +4
Speech signal cascade processing method, terminal, and computer-readable storage medium
ActiveUS20180286422A1Improving speech signal clarityReduce lossesSpeech analysisSignal qualityVoice communication
A method for improving speech signal intelligibility is performed at a device. A speech signal is obtained. A correspondence between the speech signal and a respective user group among different user groups having distinct voice characteristics is identified. Pre-encoding signal augmentation is performed on the speech signal with a respective pre-augmentation filtering coefficient that corresponds to the respective user group to obtain a group-specific pre-augmented speech signal. The device encodes the pre-augmented speech signal for subsequent transmission through the voice communication channel. An encoded version of the pre-augmented speech signal has reduced loss of signal quality as compared to an encoded version of the speech signal that is obtained without the pre-encoding signal augmentation.
Owner:TENCENT TECH (SHENZHEN) CO LTD
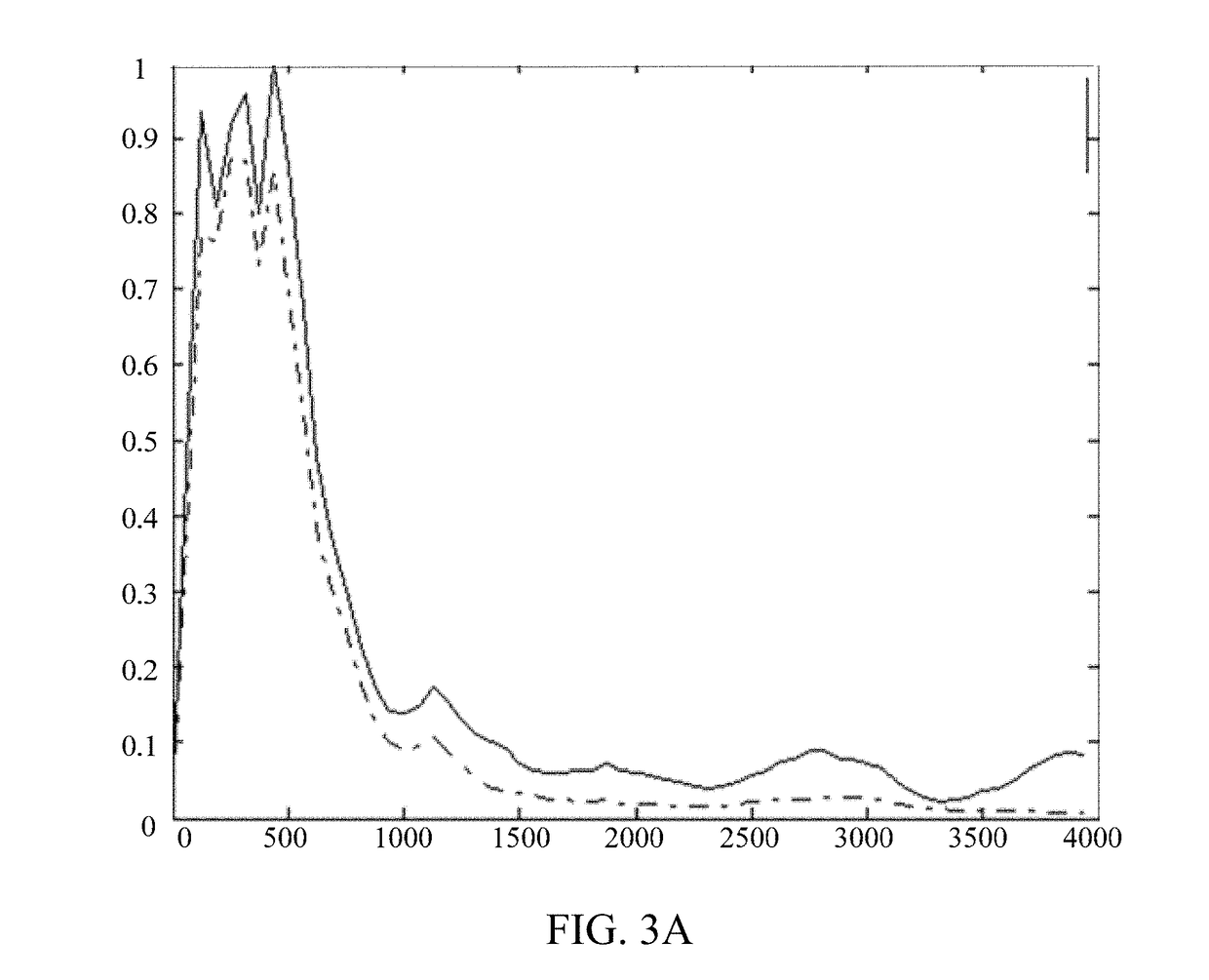
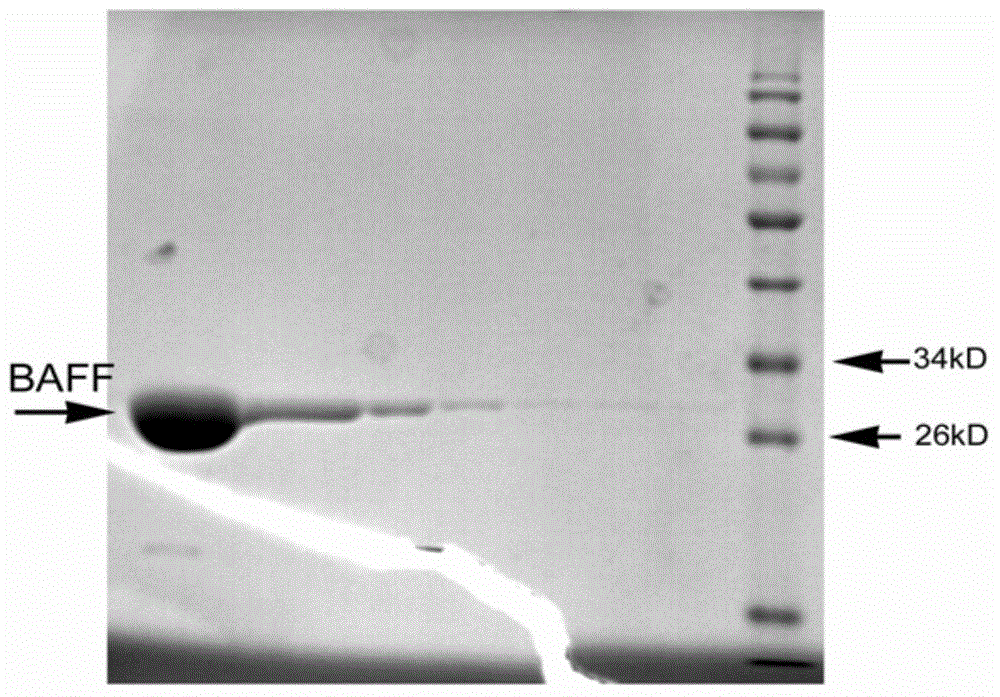
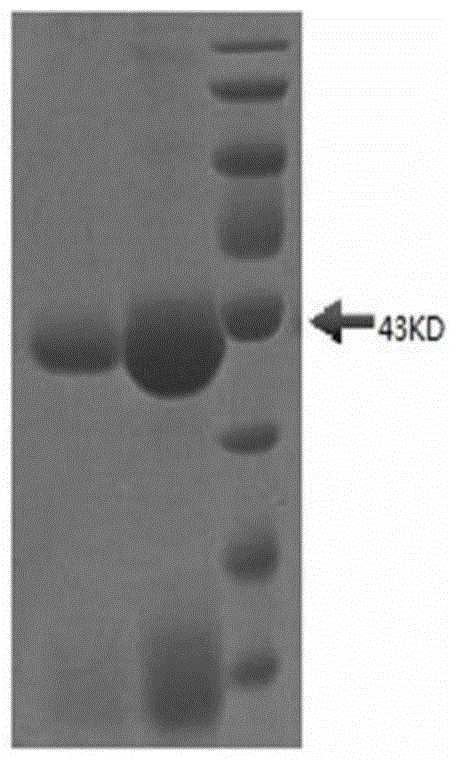
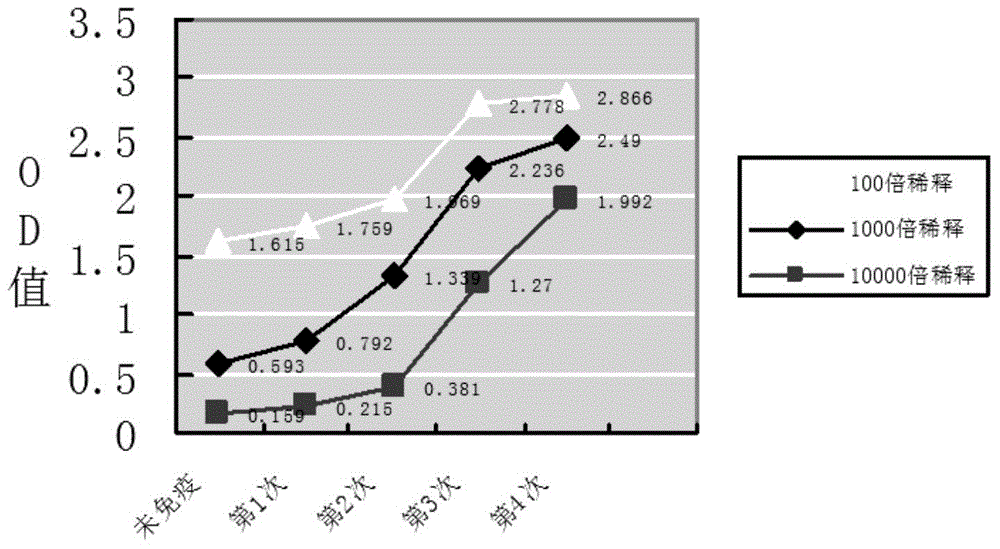
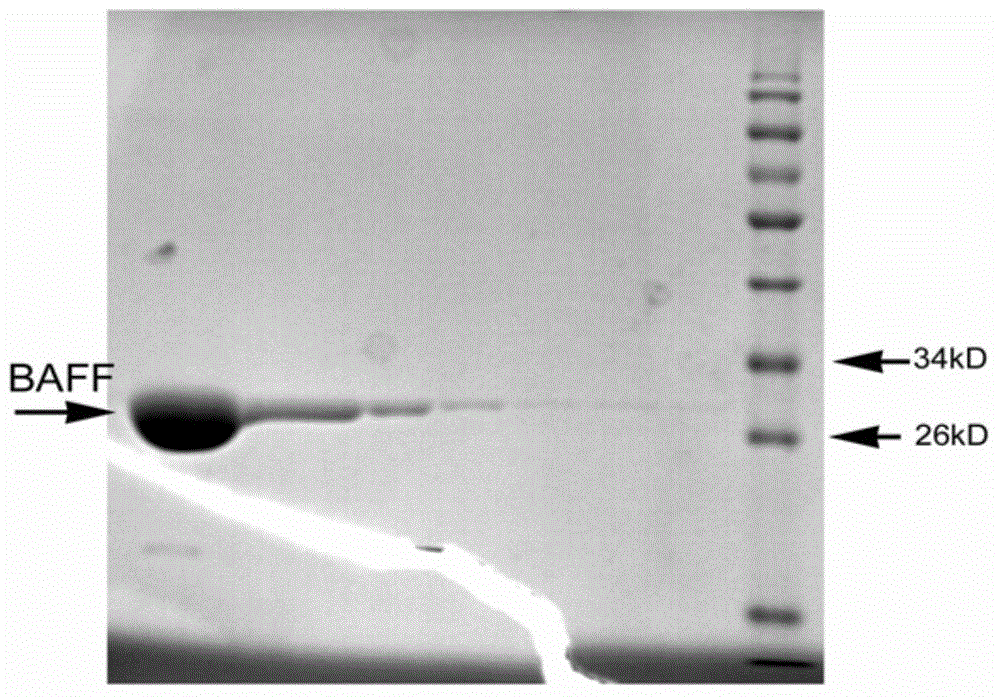
Nanometer antibody for resisting B cell growth stimulating factor and use thereof
The invention discloses a nanometer antibody for resisting a B cell growth stimulating factor and a use thereof and belongs to the field of biotechnology. The nanometer antibody for resisting a B cell growth stimulating factor has an amino acid sequence shown in the formula of SEQ ID NO. 1 in the sequence table. Through screening of multiple nanometer antibodies with high activity and a latent neutralising capacity, the nanometer antibody 52 for resisting BAFF is obtained. The nanometer antibody can specifically bond with a human BAFF antigen, adjust biological activity of the BAFF antigen and related ligands, and effectively inhibit bonding of the BAFF antigen and its acceptors and production of corresponding signal cascade effects. The nanometer antibody for resisting BAFF can block combination of BAFF and its three related receptors, can substantially inhibit B cell proliferation or viability and can be used for detection and / or treatment on a plurality of BAFF expression abnormity-related diseases.
Owner:TIANJIN SHENGFA NABIOTECH
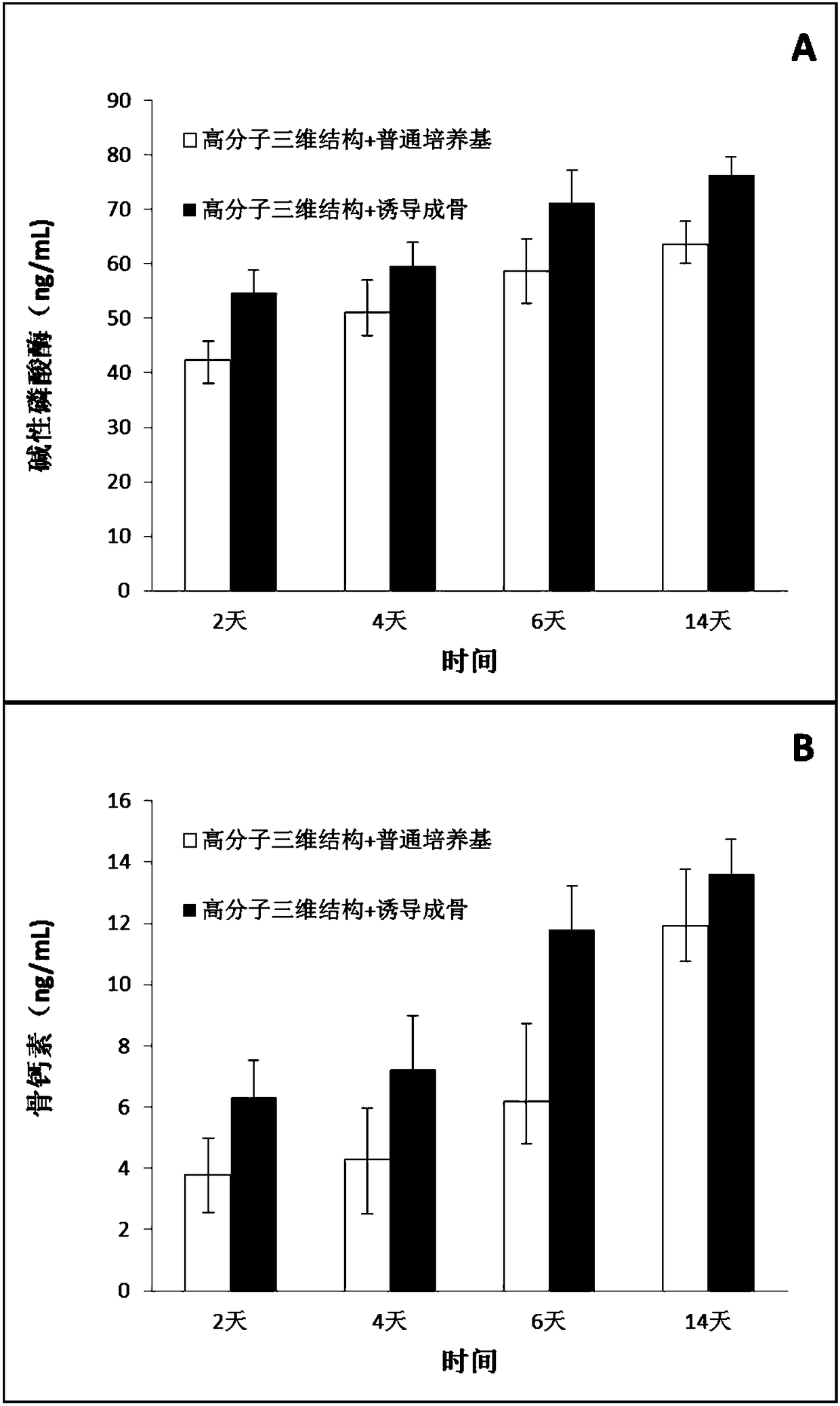
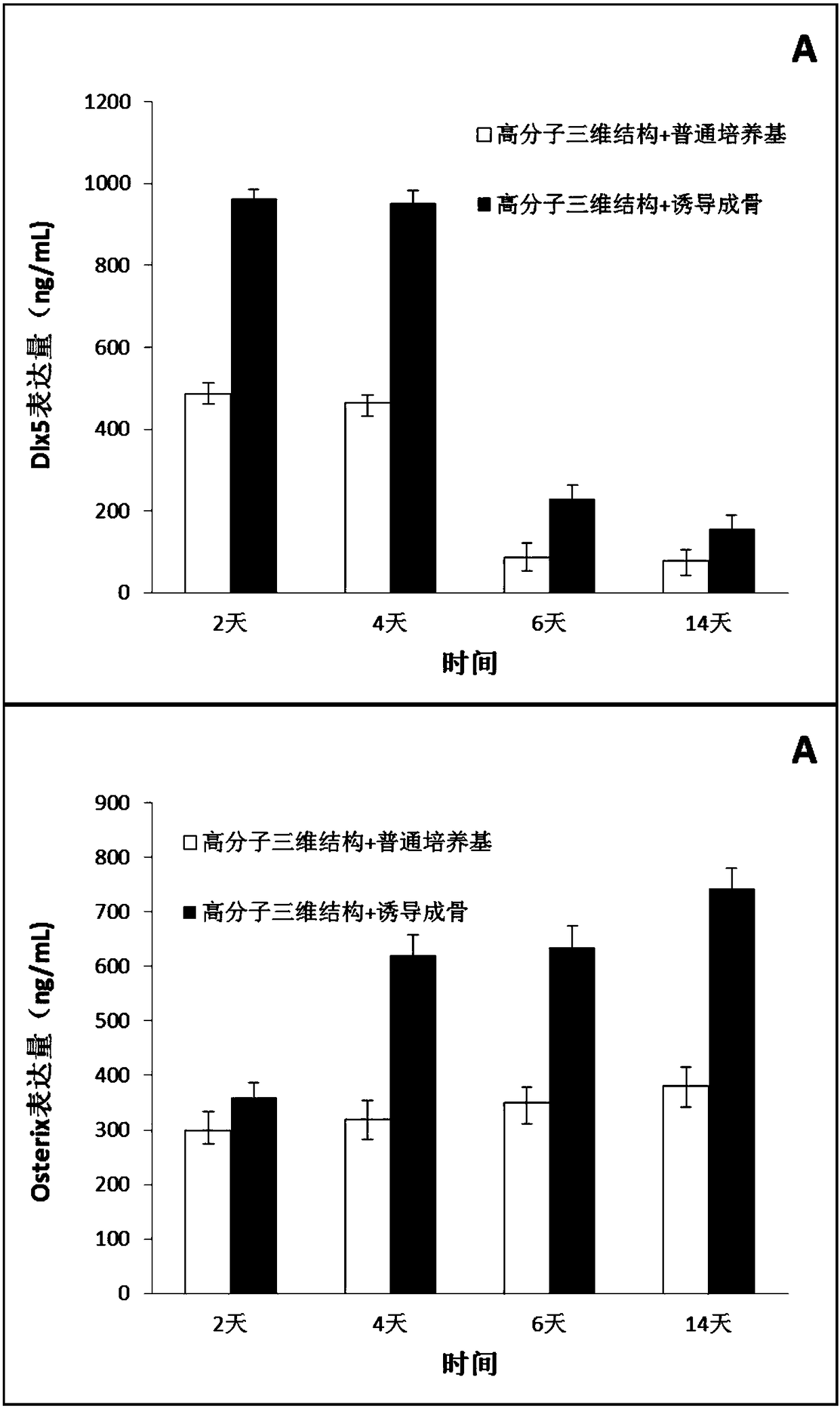
Method for inducing mesenchymal stem cells to be directionally differentiated into osteoblasts
InactiveCN108373993AReduce the impactGood osteogenic propertiesCulture processCell culture supports/coatingCell to cell signalingSignalling cascade
The invention belongs to the field of biomedical engineering, and particularly relates to a method for inducing mesenchymal stem cells to be directionally differentiated into osteoblasts. The method for inducing the mesenchymal stem cells to be directionally differentiated into the osteoblasts has the advantages that the problem of deficiency of a simple, convenient, economical, safe and effectivemethod for inducing mesenchymal stem cells to be differentiated into osteoblasts in the prior art can be solved; macromolecular three-dimensional structures are constructed, appropriate micro-environments are provided, collagen composition and mediation intercellular signal cascade amplification are adopted, culture is carried out on the mesenchymal stem cells in osteogenic induction culture media, accordingly, expression of osteogenesis-related transcription factors can be strengthened, and the mesenchymal stem cells can be promoted to be differentiated into the osteoblasts.
Owner:重庆斯德姆生物技术有限公司
Compositions and methods for regulating cancer-related signaling pathways
InactiveUS20140348744A1Improve survivabilityLibrary screeningImmunoglobulins against cell receptors/antigens/surface-determinantsADAMTS ProteinsTreatment targets
Owner:PINSKI JACEK K
Compounds and methods of treating ocular disorders
A method of treating an ocular disorder in a subject includes administering to the subject a therapeutically effective amount of an agent that modulates at least one target in a signaling cascade associated with light induced retinal degeneration, aberrant all-trans-retinal accumulation, and / or generation of reactive oxygen species (ROS).
Owner:CASE WESTERN RESERVE UNIV
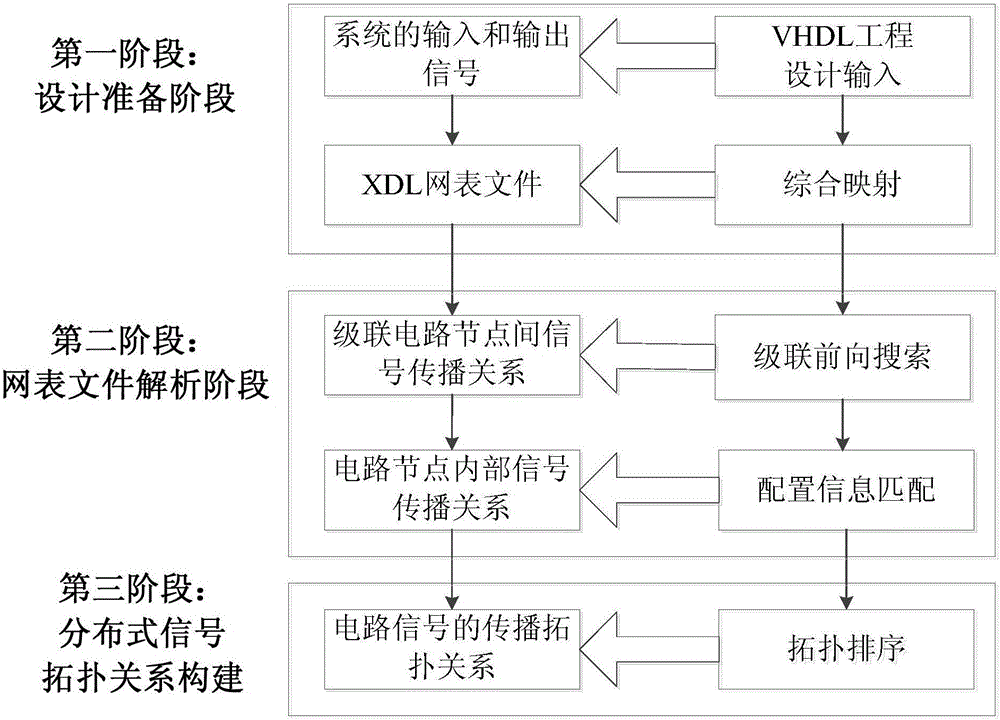
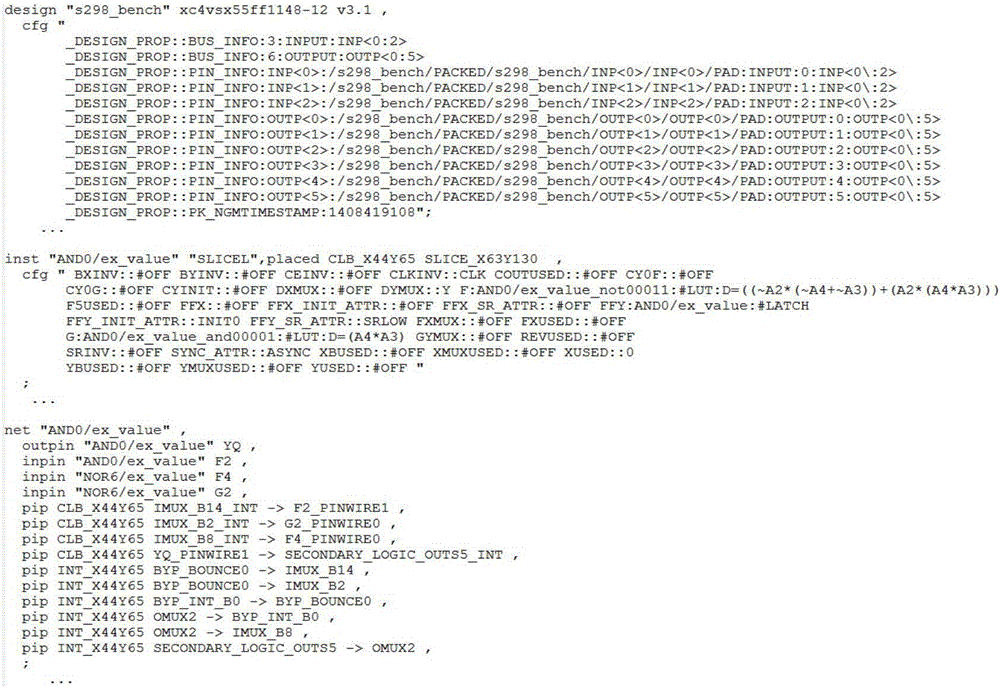
Distributed signal topological relation construction method used for analyzing single-particle and soft-error fault propagation
ActiveCN106326553AImproving the accuracy of single event soft error reliability analysisImprove reliability analysis accuracySpecial data processing applicationsSignalling cascadeAnalysis method
The invention relates to a distributed signal topological relation construction method used for analyzing single-particle and soft-error fault propagation and belongs to the technical field of evaluation of single-particle and soft-error reliability in a system. The method comprises the following steps: based on circuit nodes and corresponding inter-signal cascaded information under hardware resource construction in XDL (Xilinx Design Language) net list information, providing a cascaded forward search analysis method of signal cascaded information between the circuit nodes, constructing a directed propagation topological relation between the circuit nodes in the system and finishing circuit signal propagation relation analysis between hardware resources with different types; meanwhile, correlating circuit connection reflected according to an XDL net list and control configuration bit information. According to the method provided by the invention, a configuration information matching rule is provided, and an internal signal propagation path under resource mapping of the circuit nodes is analyzed and constructed; the circuit signal propagation relation analysis under the hardware resources with the specific type is finished.
Owner:XIAN INSTITUE OF SPACE RADIO TECH
Cell-specific signaling biomarker analysis by high parameter cytometry; sample processing, assay set-up, method, analysis
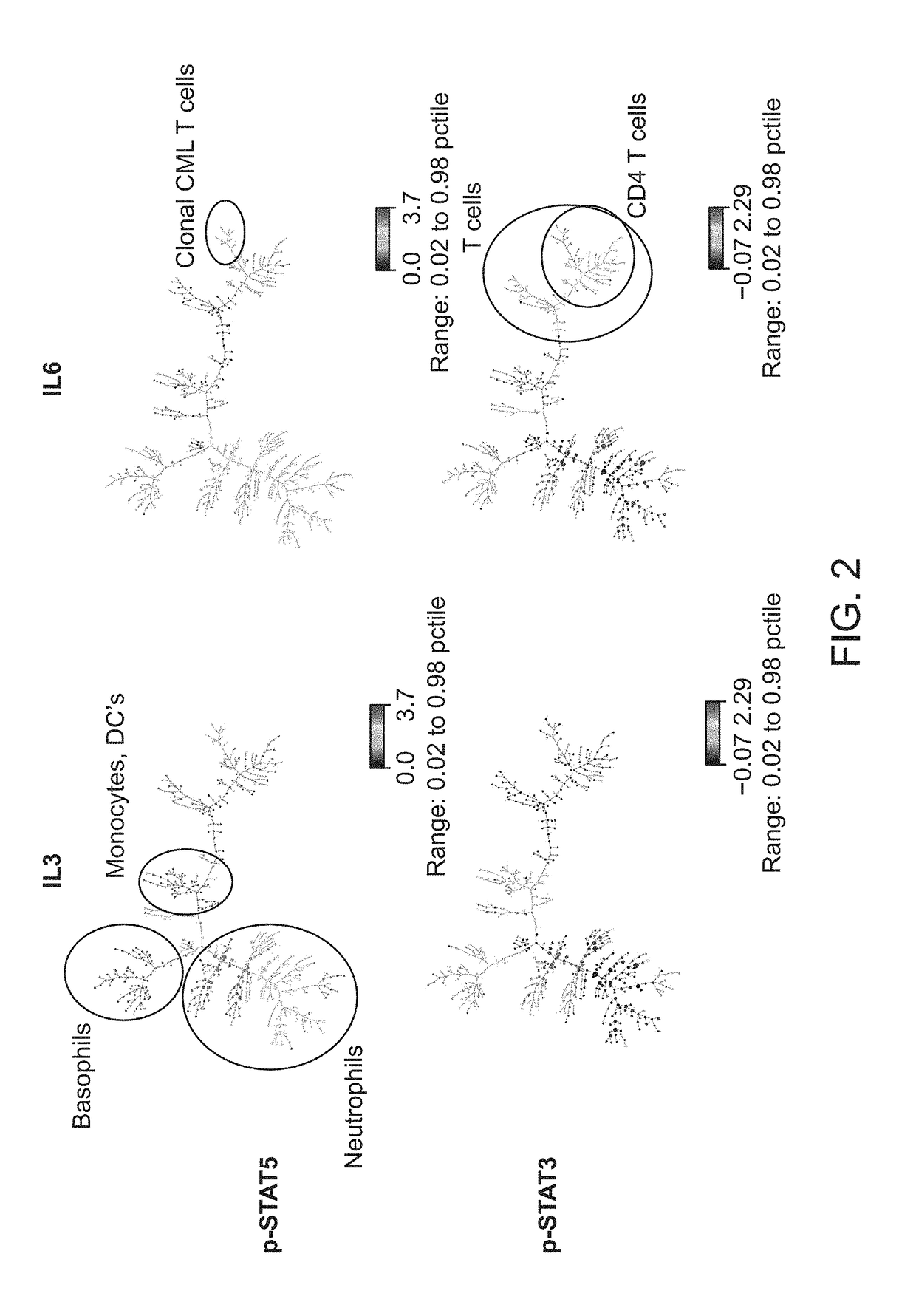
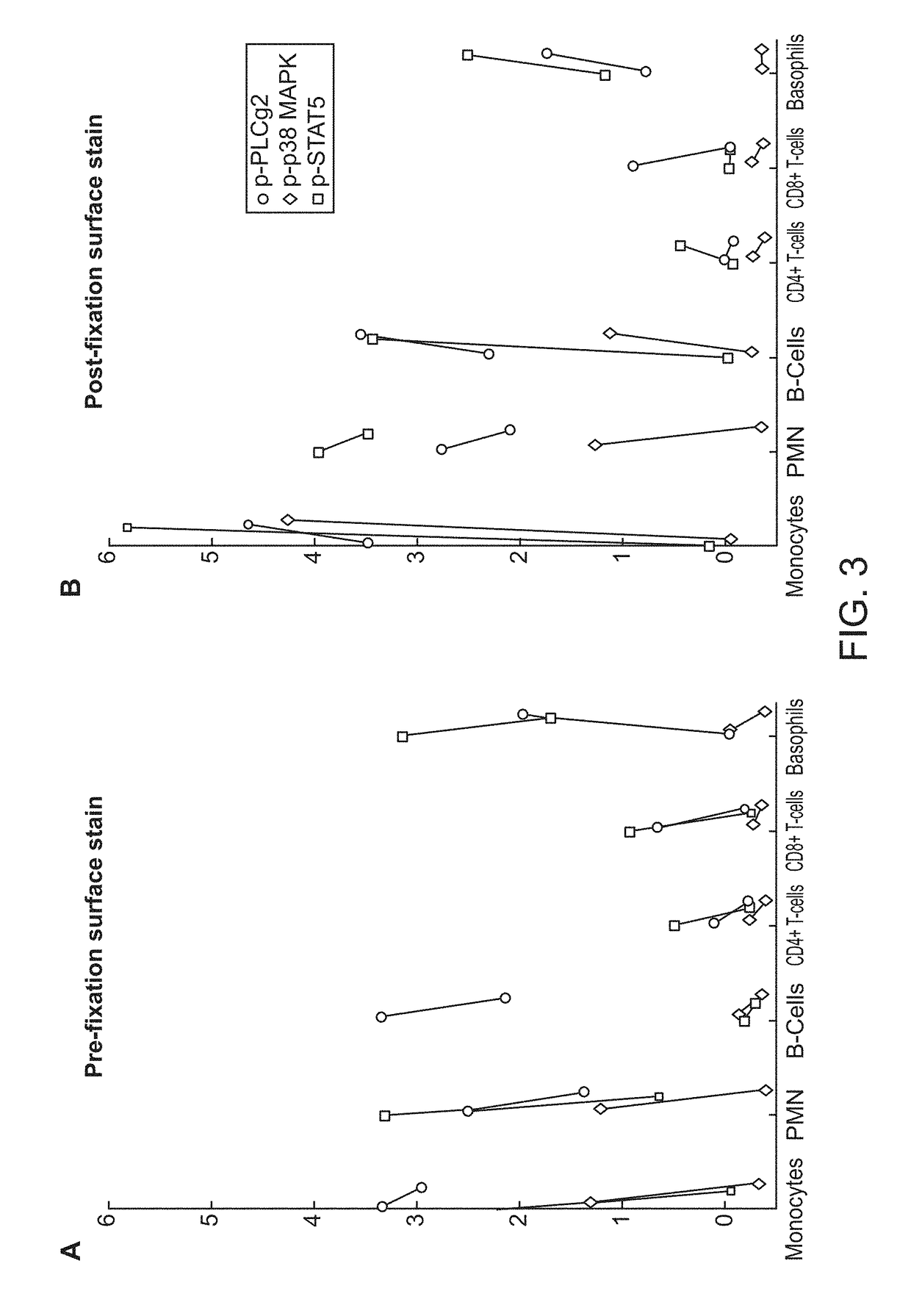
ActiveUS20180252708A1Facilitate data interpretationEasy to explainDisease diagnosisIndividual particle analysisMarker selectionProgenitor cell
The present invention recognizes that current clinical laboratory testing methods for multiparametric single cell analysis are limited to analysis of intact live cells, and are insufficient for identification of signaling activation profile defining certain cell types, including but not limited to neoplastic and immunologically activated cell subsets. One aspect of the present invention generally relates to marker selection in panels to include proteins routinely assessed in standard FCM, while preferably also incorporating markers for surface receptor proteins within activated signaling cascades. A further aspect of the present invention generally relates to panel design for the following indications in neoplastic and non-neoplastic clinical applications as examples of the technology: (a) identification of CML progenitor cell subsets in the setting of disease recurrence after treatment discontinuation or relapse due to treatment resistance, and (b) characterization of activated basophils to predict the severity of an allergic response. Another aspect of the present invention generally relates to methods to measure levels of surface and IC biomarkers in separate or combined assays for robust characterization of each or select cell compartment, and data analysis based on results from each or all method(s) used for optimal detection of the markers. A further aspect of the present invention generally relates to the identification and profiling of cell subpopulations based on analysis of surface markers including those associated with lineage and maturation of cell types and receptor proteins, and the corresponding IC phosphoproteins including those in activated signaling cascades to predict certain disease states or response to treatment.
Owner:DEEPATH MEDICAL
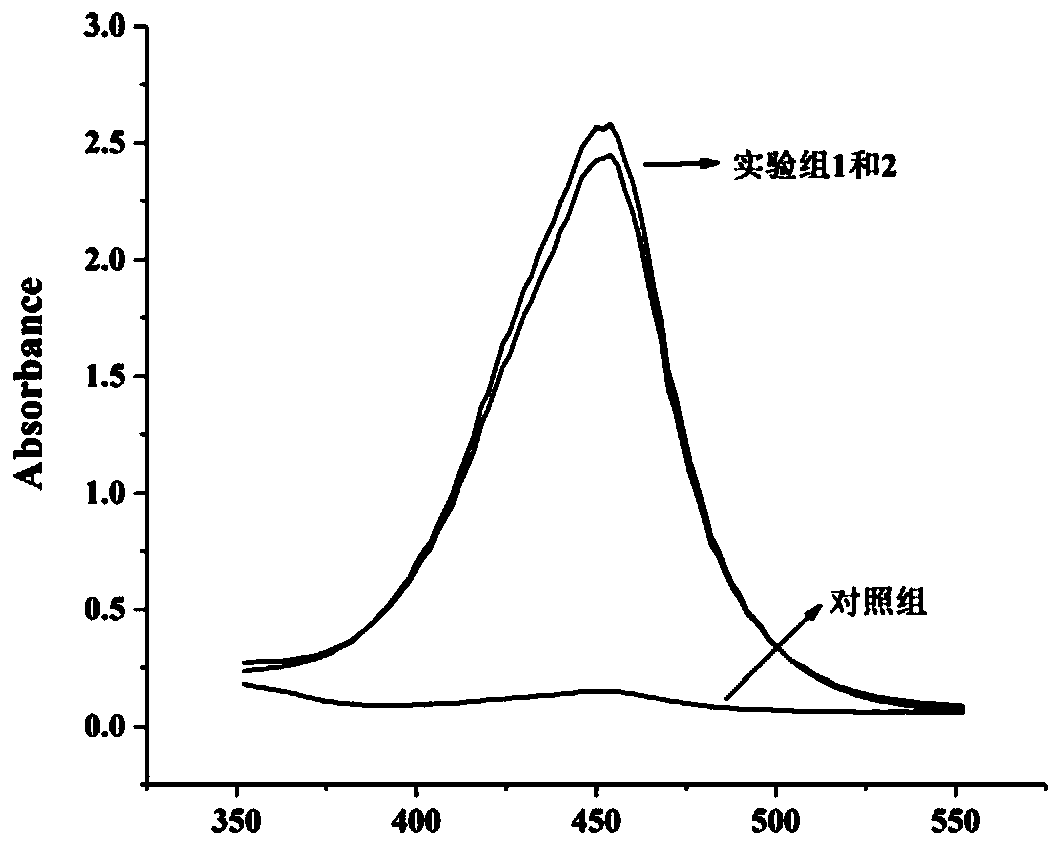
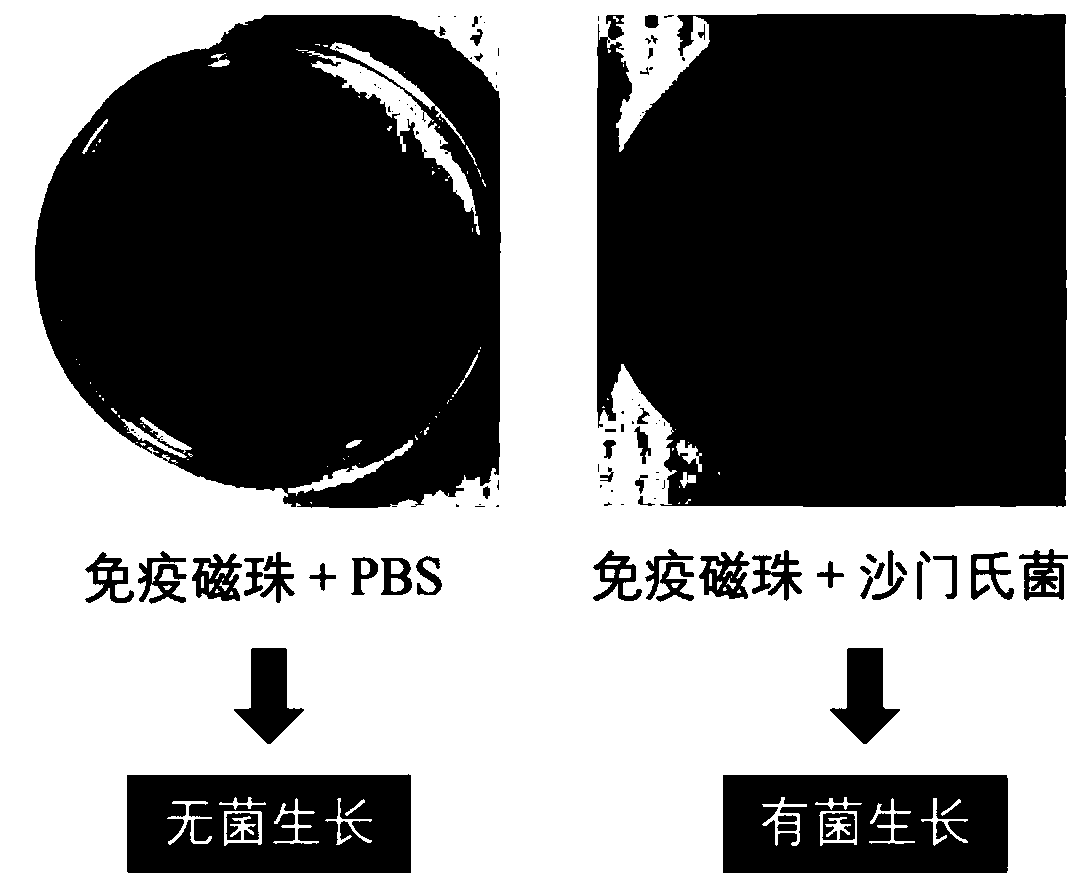
Highly-sensitive and rapid method for detecting food-borne pathogenic bacteria based on signal cascade double-amplification system
The invention discloses a highly-sensitive and rapid method for detecting food-borne pathogenic bacteria based on a signal cascade double-amplification system. The method mainly comprises the following steps: (1) coupling a polyclonal / monoclonal antibody of pathogenic bacteria to the surface of a magnetic bead to obtain an immunomagnetic bead; (2) mixing the immunomagnetic bead with a sample containing food-borne pathogenic bacteria, and specifically capturing the pathogenic bacteria through antigen-antibody reaction; (3) initiating a hybridization chain reaction by using the pathogenic bacteria aptamer modified by the initiation sequence and a DNA hairpin structure probe to obtain an HCR product (double-stranded DNA with a notch); and (4) adding Raman signal molecules and a Raman substrate into the HCR product, and carrying out corresponding SERS determination to realize cascade amplification and output of signals. The method has great significance in guaranteeing food safety and preventing food-borne diseases, and has great application potential for other biological analysis.
Owner:EAST CHINA UNIV OF SCI & TECH
Signal Repeater System
InactiveUS20080069025A1Secures repeatabilityReduce mutual interferenceInterconnection arrangementsFrequency-division multiplex detailsAnalog signalEngineering
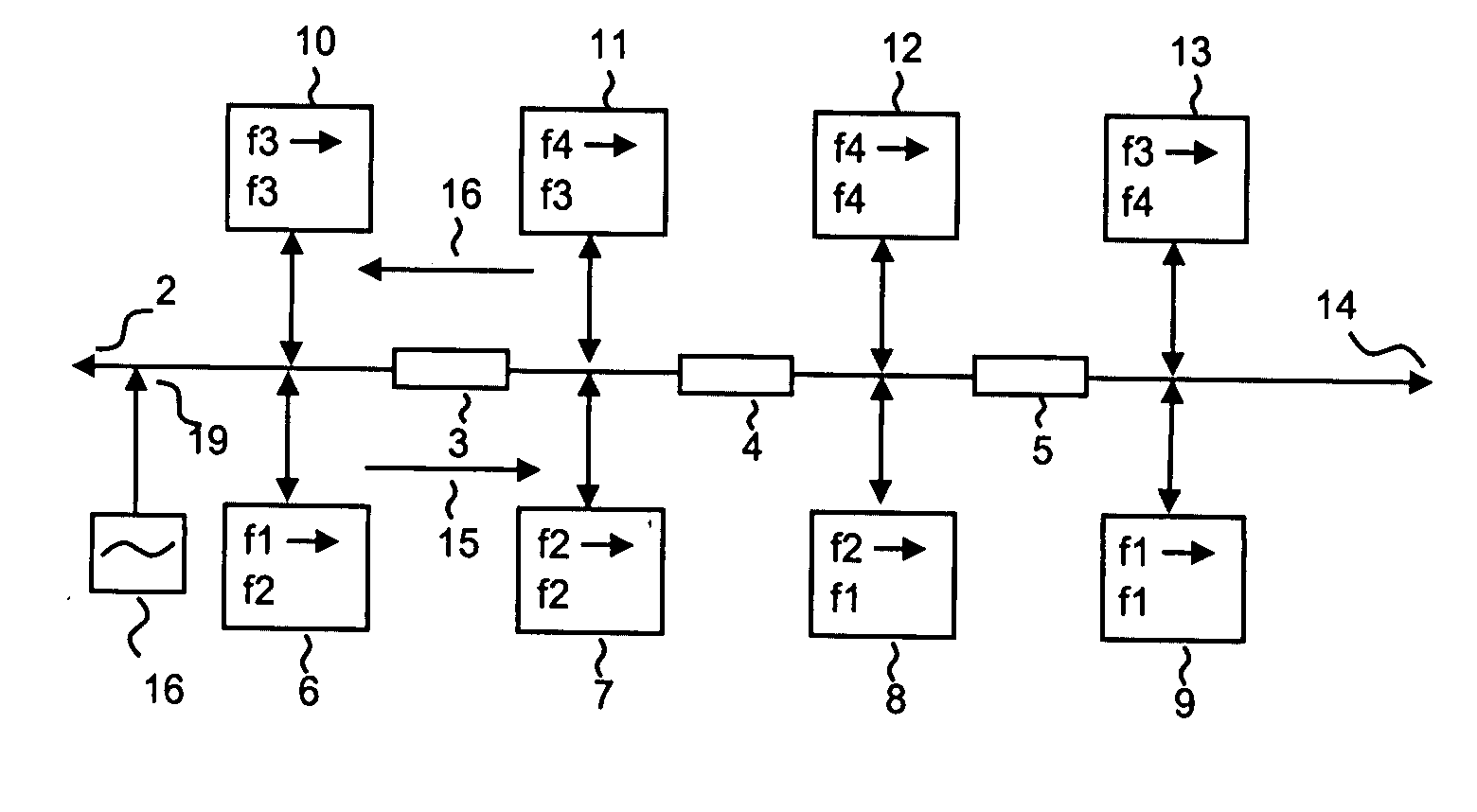
Analogue signal repeater system, (1) where frequency converting repeaters (6-9, 10-13) of super-heterodyne or superregenerative type realised with any of discrete semiconductors, MMIC semiconductors, ASIC semiconductors are applied to optimize signal dynamics by avoiding echo between repeaters (6-9, 10-13) and where each information channel (15, 16) in the system only needs two frequency bands, where each second repeater (7, 9, 12, 10) of the signal cascade (2, 14) repeating the signals within the same frequency band to increase isolation against interference between repeaters and against reflections and signal echo.
Owner:VAVIK GEIR MONSEN
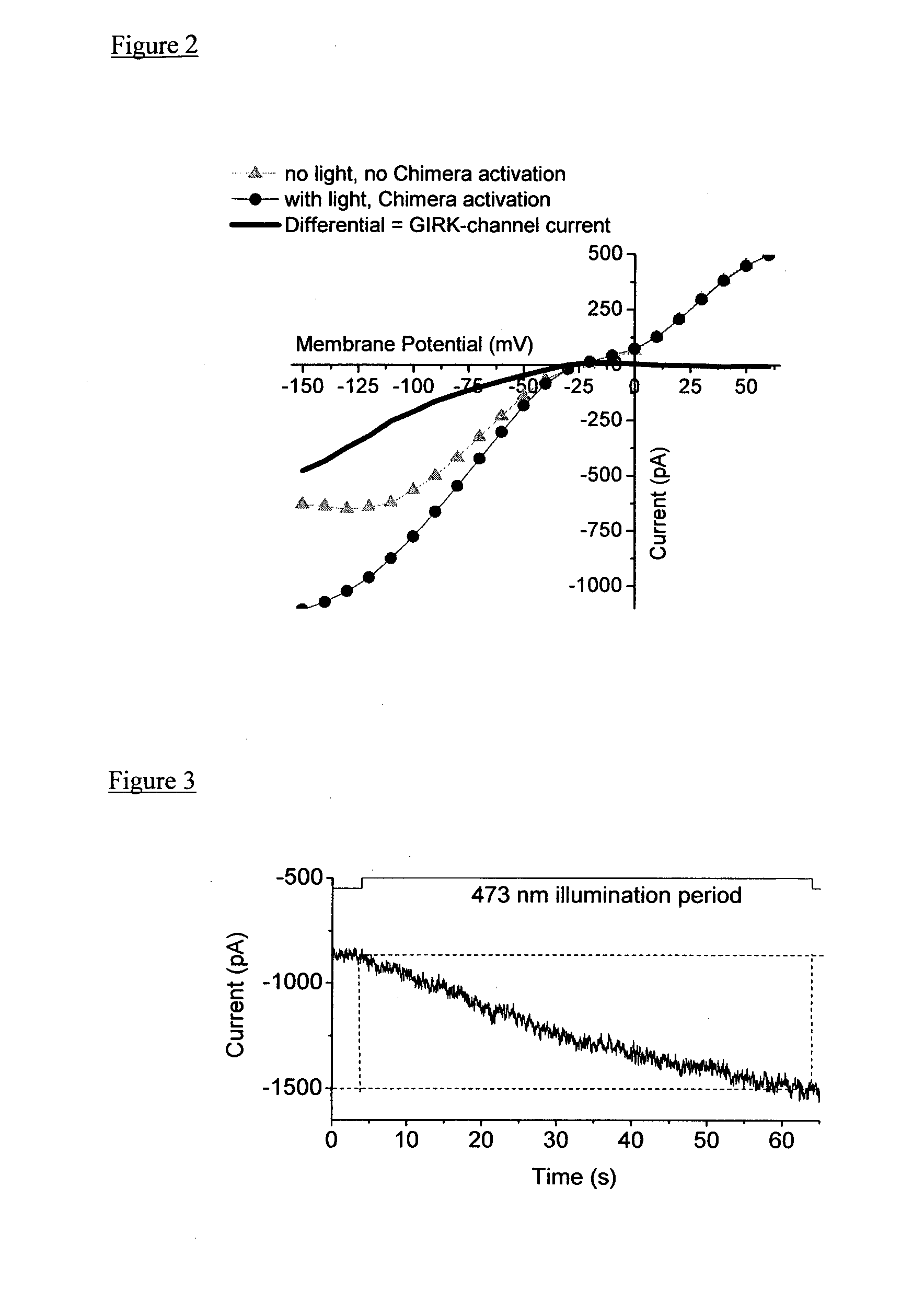
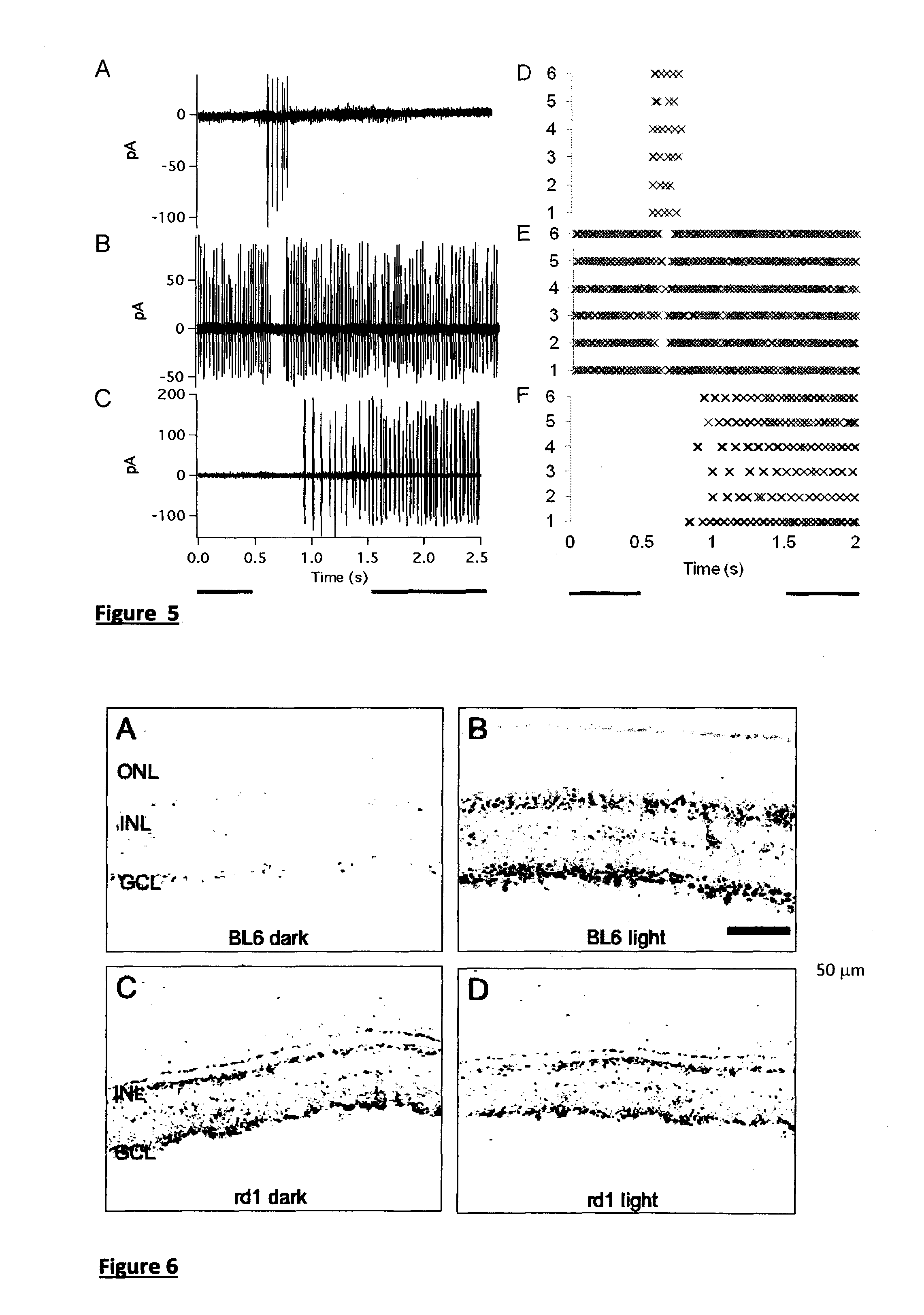
Light-Sensitive Chimeric GPCR Protein
ActiveUS20140171376A1Increase signal differenceHigh light sensitivityPolypeptide with localisation/targeting motifSenses disorderIntracellular signaling cascadeIntracellular signalling
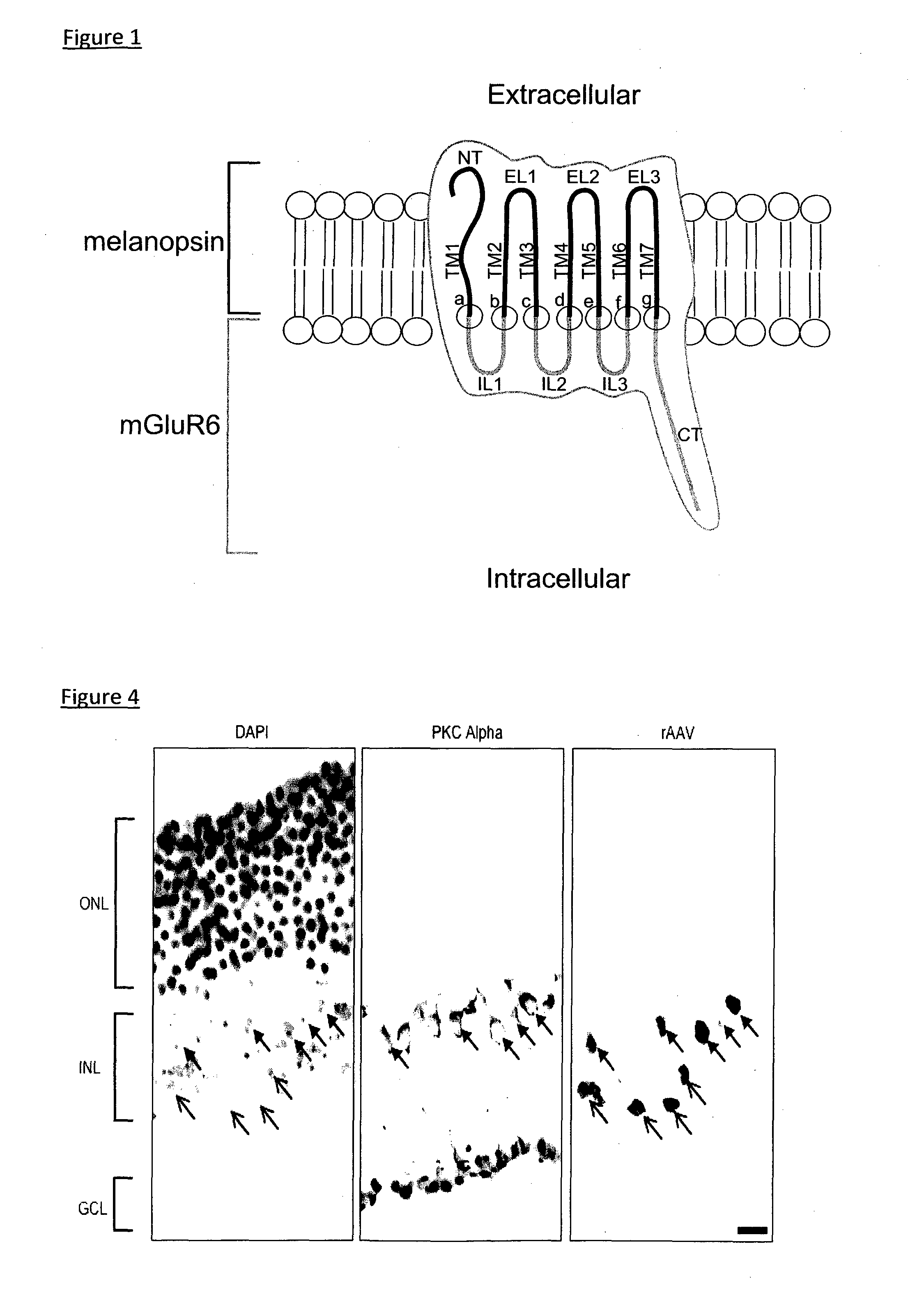
A light-sensitive chimeric protein comprising domains from at least two members of the G-protein-coupled-receptor (GPCR) protein super family, which are fused to yield a light-sensitive GPCR chimera capable of coupling a light signal to the signaling cascade of the metabotropic glutamate receptor 6 (mGluR6) is provided for medical therapy and for the manufacture of medicaments for improving vision, in particular for treating loss of vision resulting from retinal photoreceptor degeneration. A first of the at least two GPCR family members contributes domains which mediate the light-sensitivity to the chimeric light-sensitive GPCR protein. This first member belongs to the family of light-sensitive GPCR proteins also called photopigments, and in some embodiments this light-sensitive GPCR protein is melanopsin, in particular human melanopsin. A second of the at least two GPCR family members is mGluR6, which contributes domains for coupling the light signal to the intracellular signalling cascade of mGluR6, which is a native component of the cell membrane of ON-bipolar cells in the inner retina.
Owner:NOVARTIS AG
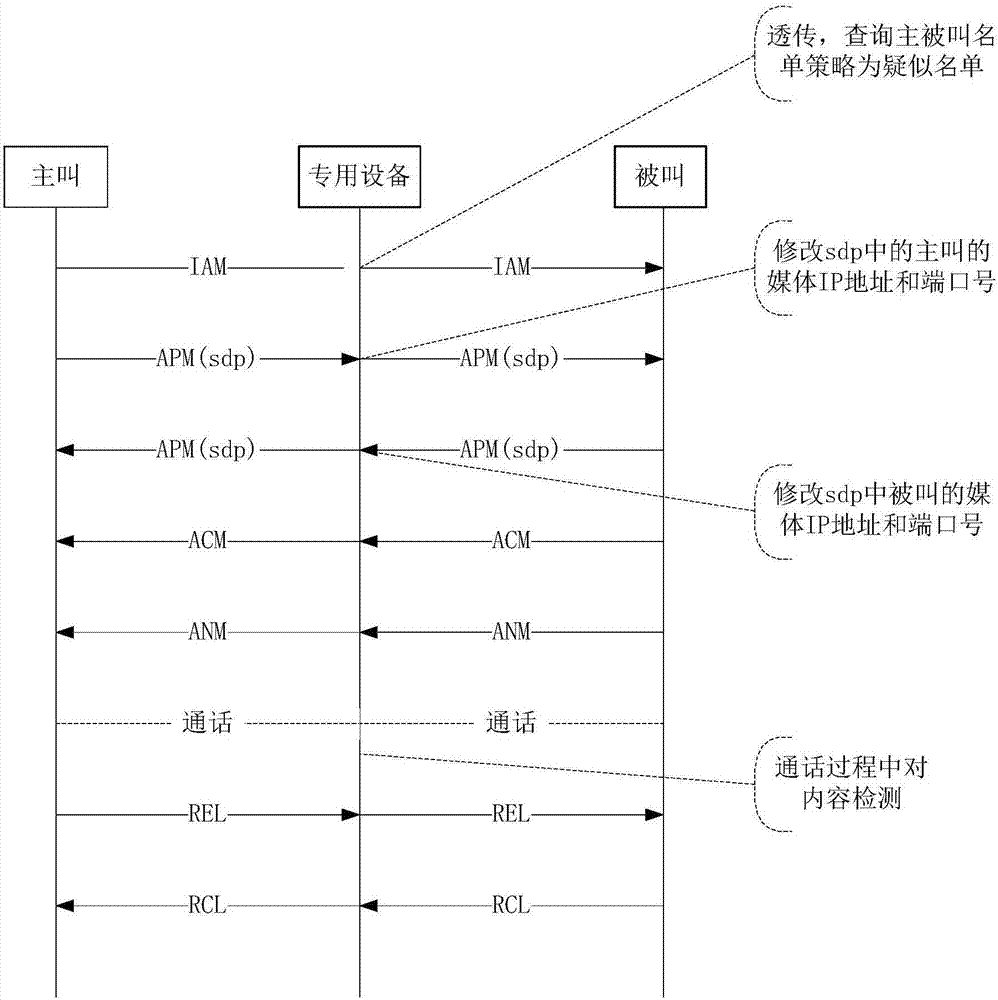
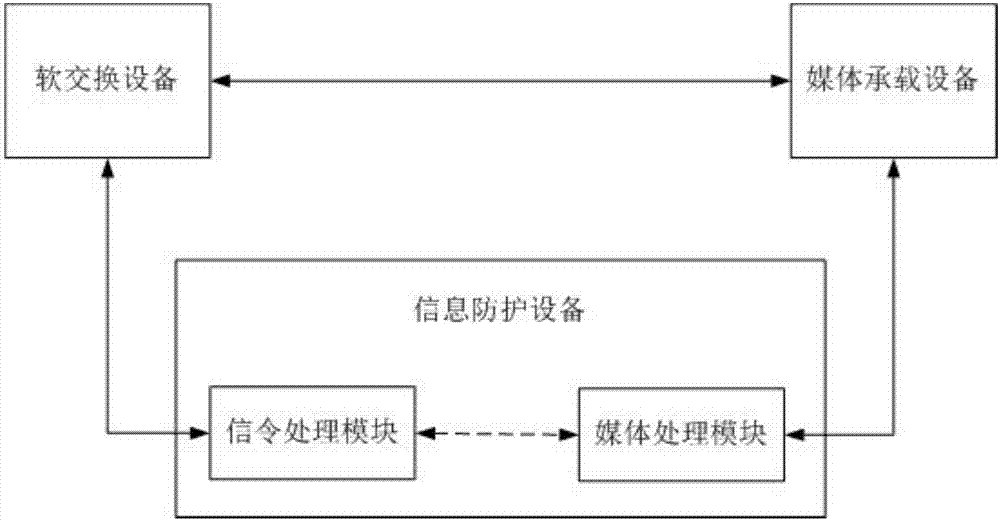
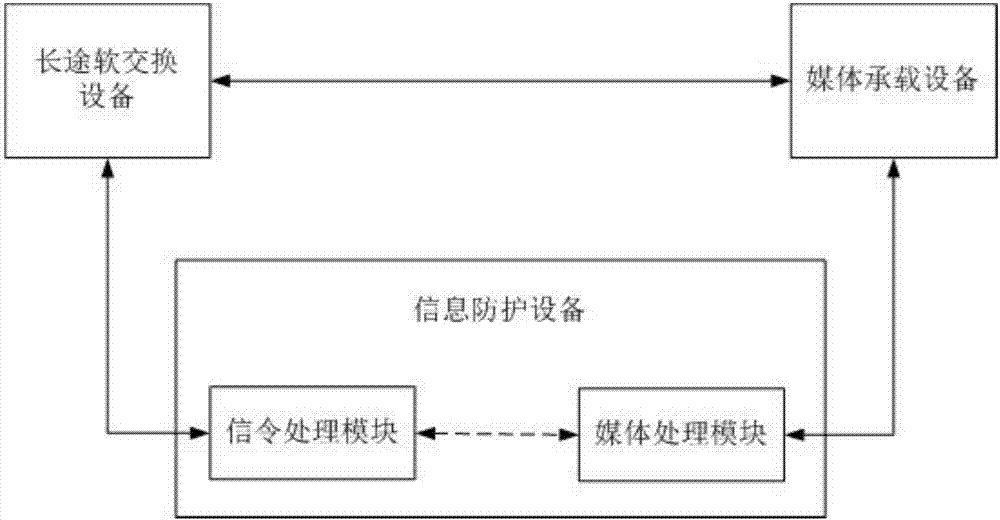
Signal cascading media redirection fraud call prevention access system and method
InactiveCN107395905AReach the purpose of redirectionSolve the problem of access detectionAutomatic exchangesTransmissionSoftswitchNetwork media
The invention discloses a signal cascading media redirection fraud call prevention access system. The system comprises media carrying equipment, information protection equipment and soft exchange equipment, wherein the media carrying equipment communicates with a whole-network media gateway; the information protection equipment is used for detecting signals and processing and forwarding bidirectional media streams; the soft exchange equipment is used for continuing the signals and / or the media streams; the media carrying equipment is in a communication connection with the soft exchange equipment; the information protection equipment accesses side ends of the media carrying equipment and the soft exchange equipment respectively; if the information protection equipment finds a fraud call suspicious call during detection of signals flowing inwards through the media carrying equipment, bidirectional media stream paths of a calling party and a called party are redirected to pass through the information protection equipment, and bidirectional media streams are processed and forwarded through the information protection equipment; and if the fraud call suspicious call is not found, the media stream path is not changed. The invention also provides a method corresponding to the system.
Owner:ZHUHAI GAOLING INFORMATION TECH COLTD
Double specific ERB-B antibody and its use in treating tumor
InactiveCN100408097CImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsEpitopeBinding site
The invention relates to novel bispecific antibodies and their use in tumor therapy. The novel antibodies have the ability to bind to ErbB receptors, preferably ErbB1 receptors, which are overexpressed on many cancer tissues. Since the different specificities of the antigen-binding sites are directed to different epitopes within the binding domain of same or different ErbB receptors, these antibodies are more effective with respect to inhibition and down-regulation of the ErbB receptor and the corresponding signaling cascade.
Owner:MERCK PATENT GMBH
Composition and method for preventing or treating a tauopathy
ActiveUS20160031978A1Nervous disorderPeptide/protein ingredientsBiological activationSignalling cascade
The present invention is a composition and method for the prevention and treatment of a tauopathy. The composition of the invention includes N-terminal amino acid residues of the tau protein, which have been identified as being involved in toxic activation of a PP1 / GSK3 signaling cascade and inhibition of fast axonal transport in human tauopathies.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS +1
Nanometer antibody for resisting B cell growth stimulating factor and use thereof
The invention discloses a nanometer antibody for resisting a B cell growth stimulating factor and a use thereof and belongs to the field of biotechnology. The nanometer antibody for resisting a B cell growth stimulating factor has an amino acid sequence shown in the formula of SEQ ID NO. 1 in the sequence table. Through screening of multiple nanometer antibodies with high activity and a latent neutralising capacity, the nanometer antibody G3 for resisting BAFF is obtained. The nanometer antibody can specifically bond with a human BAFF antigen, adjust biological activity of the BAFF antigen and related ligands, and effectively inhibit bonding of the BAFF antigen and its receptors and production of corresponding signal cascade effects. The nanometer antibody for resisting BAFF can block combination of BAFF and its three receptors, can substantially inhibit B cell proliferation or viability and can be used for detection and / or treatment on a plurality of BAFF expression abnormity-related diseases.
Owner:SHENZHEN PREGENE BIOPHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com