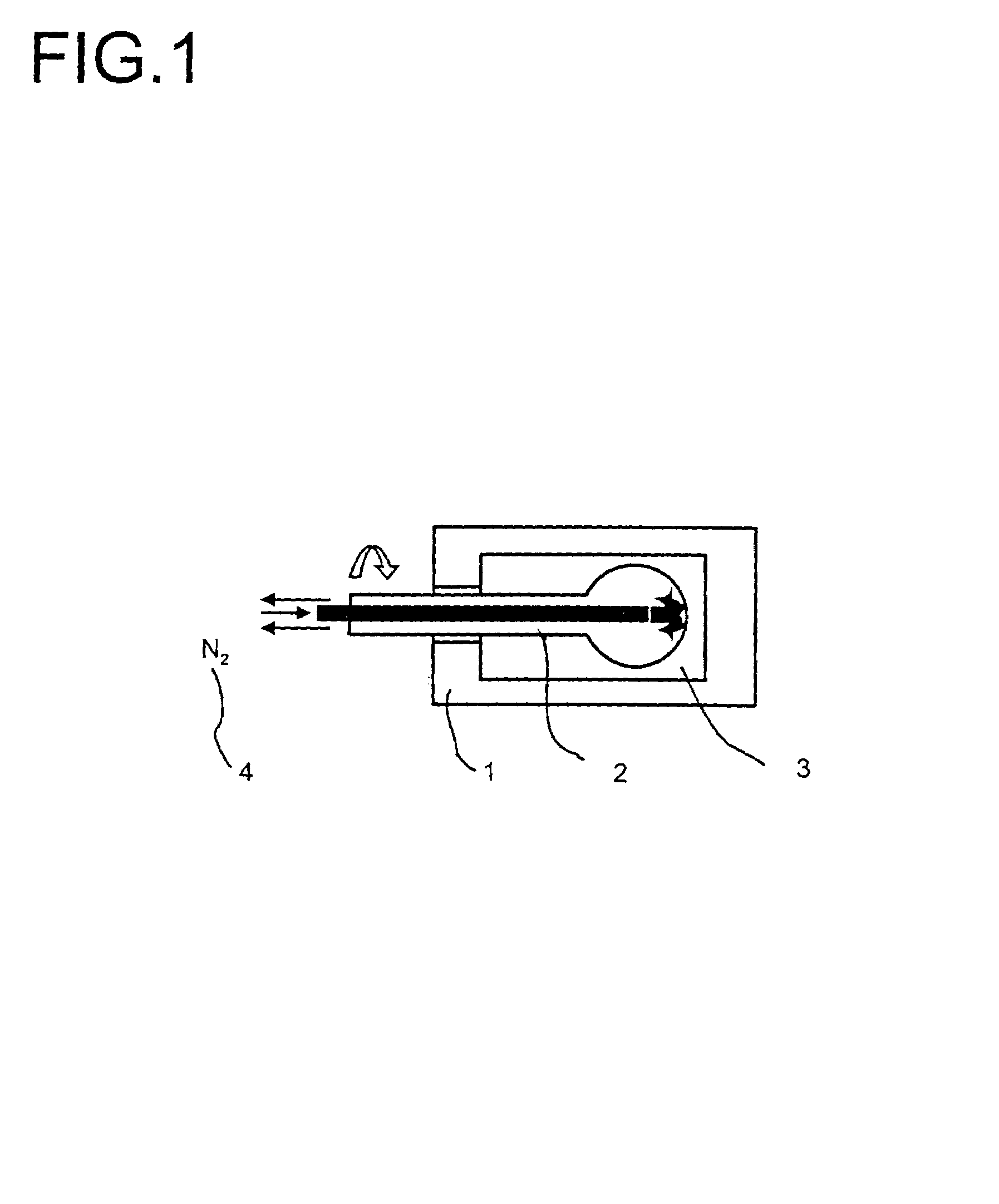
Multimetal oxide materials
a technology of multimetal oxide and oxide materials, applied in the direction of catalyst activation/preparation, metal/metal-oxide/metal-hydroxide catalysts, physical/chemical process catalysts, etc., can solve the problems of not answering the question and the quality of multi-metal oxide materials is not completely satisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0232]Similar to comparative example 1. However, the powder resulting after the milling in the Retsch mill was stirred under reflux in 1 000 ml of a 10% strength by weight HNO3 solution for 7 hours at 70° C. The remaining solid was filtered off from the resulting suspension and washed nitrate-free with water. The filter cake was then dried overnight with air at 110° C. in a muffle furnace.
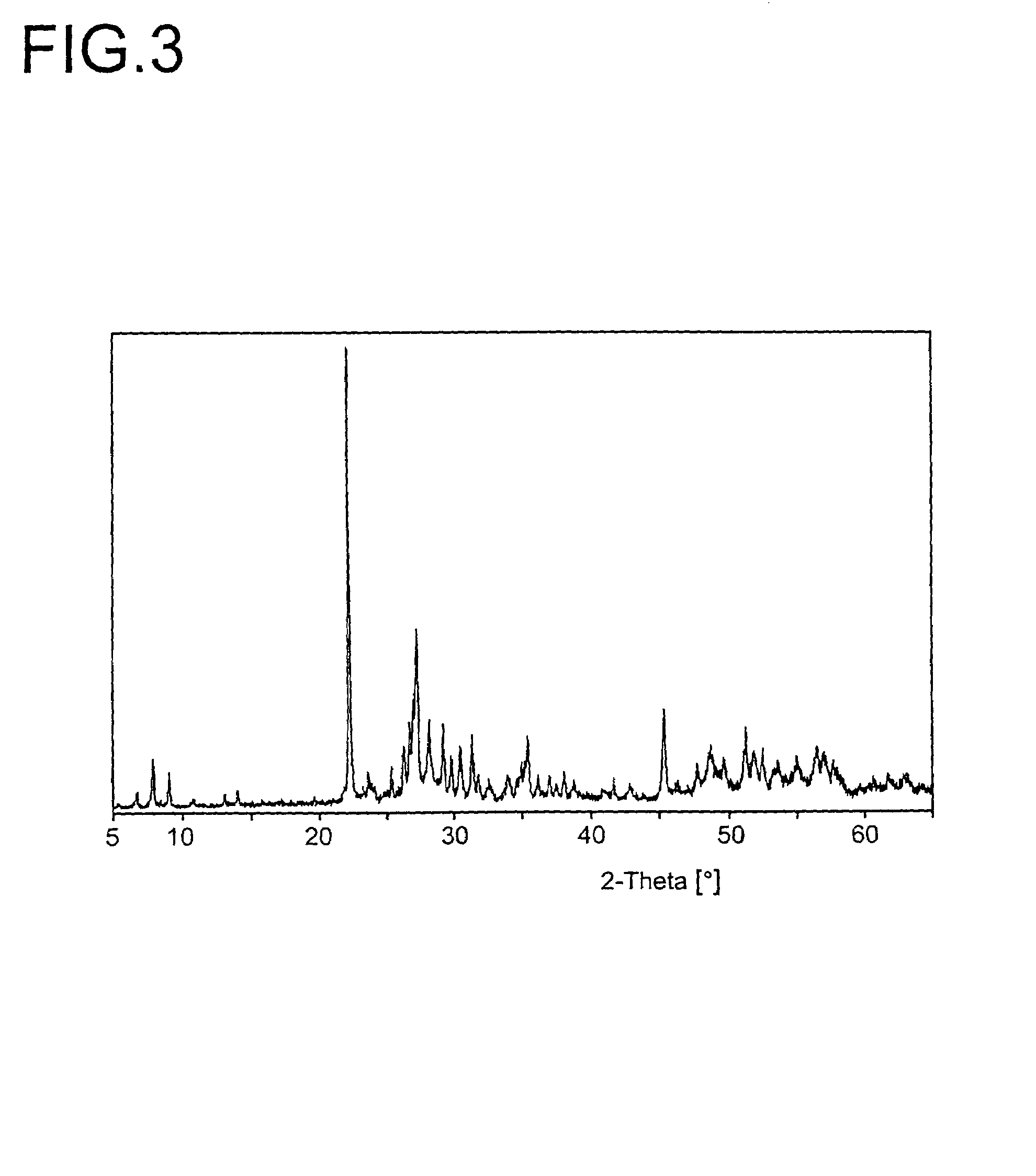
[0233]The resulting active material had the composition Mo0.1V0.29Te0.14Nb0.13Ni0.007Ox. The associated X-ray diffraction pattern is shown in FIG. 3 (R=0.71). BET=20.2 m2 / g.
[0234]It was applied to the same support as in comparative example 1, in the same manner, so that a coated catalyst E1 comprising 20% by weight of active material resulted.
example 2
[0237]Similar to example 1, but the active material from comparative example 2 was washed with aqueous nitric acid. The resulting active material had the composition Mo1.0V0.28Te0.13Nb0.13Pd0.001Ox.
[0238]The associated X-ray diffraction pattern is shown in FIG. 5 (R=0.73). BET=22.5 m2 / g. It was applied to the same substrate as in comparative example 1, in the same manner, so that a coated catalyst E2 comprising 20% by weight of active material resulted.
example 3
[0241]Similar to example 1, but the active material from comparative example 3 was washed with aqueous nitric acid. The resulting active material had the composition Mo1.0V0.29Te0.13Nb0.13Pd0.001Ox. The associated X-ray diffraction pattern is shown in FIG. 7 (R=0.74). BET=17.4 m2 / g. It was applied to the same support as in comparative example 1, in the same manner, so that a coated catalyst E2 comprising 20% by weight of active material resulted.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com