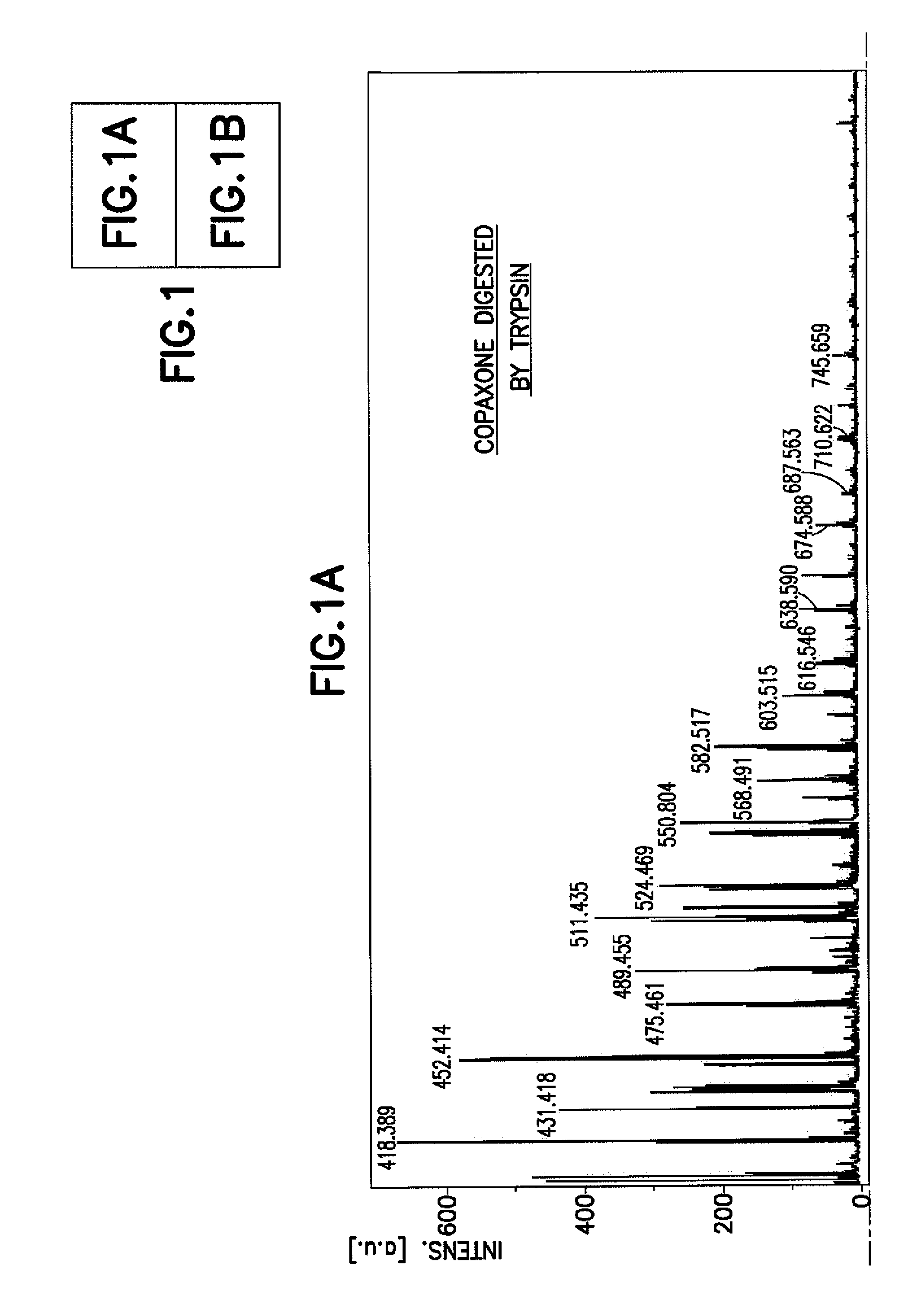
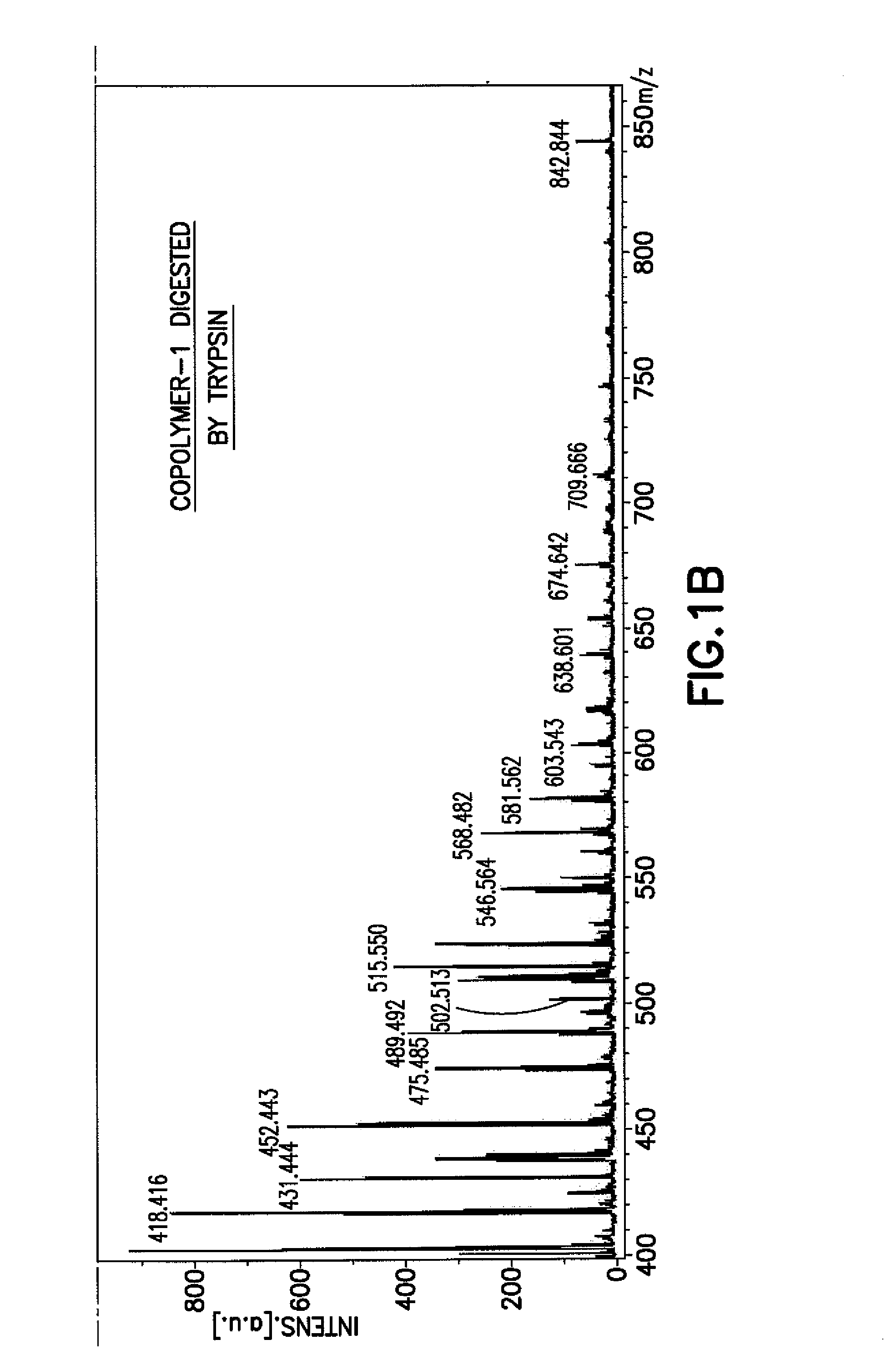
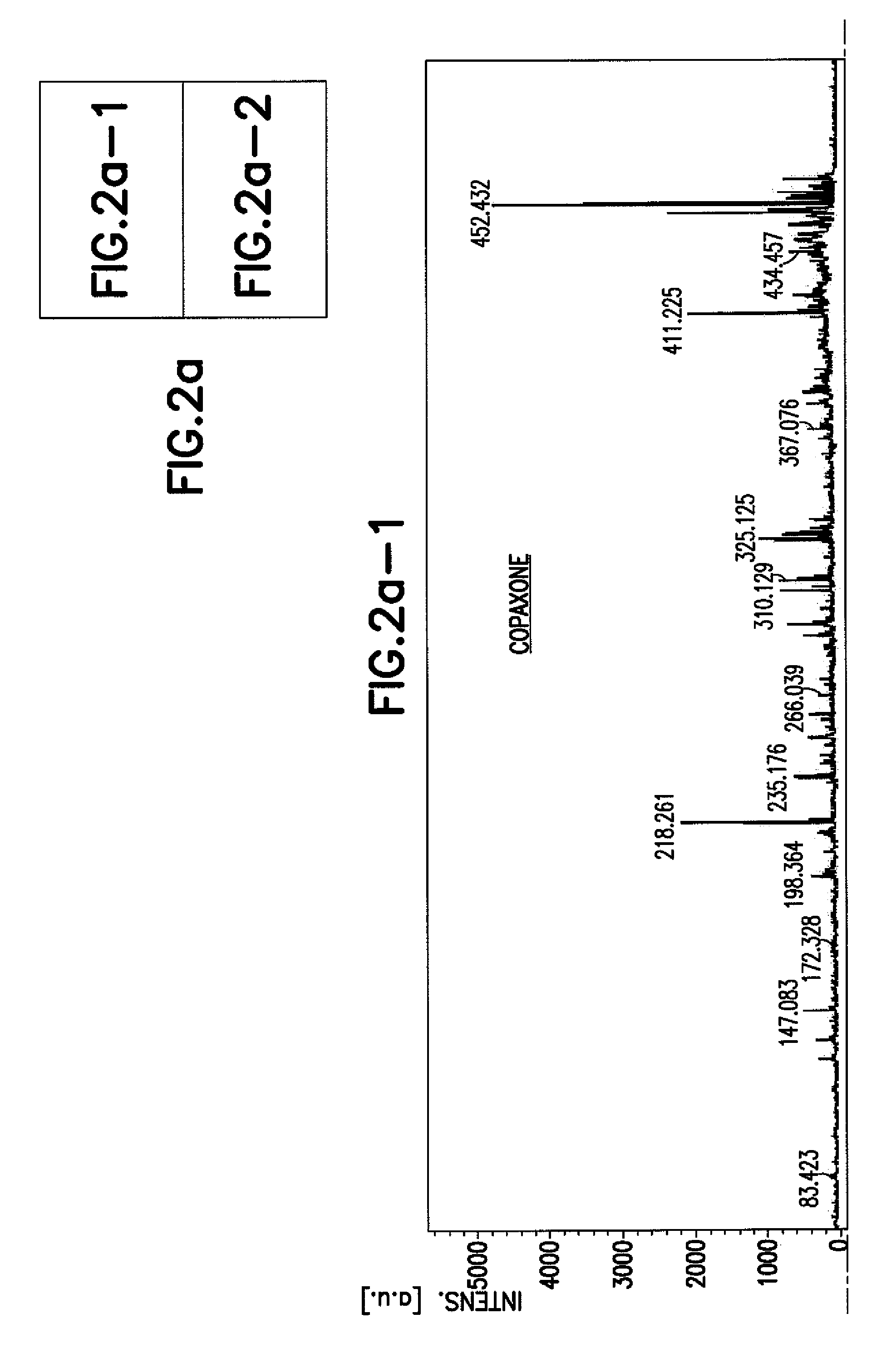
Methods for Chemical Equivalence in characterizing of complex molecules
a technology of complex molecules and chemical equivalence, which is applied in the field of analytical/statistical methods for characterizing, comparing, grouping and identifying complex molecules, can solve the problems of complex molecules such as monoclonal antibodies, peptides, polypeptide mixtures, etc., and achieves the effects of reducing the number of complex molecules, and improving the quality of complex molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Protected Copolymer-1
[0042]N-carboxyanhydride of L-alanine (4.0 g, 34.78 mmol), N-carboxyanhydride of γ-benzyl L-glutamate (3.0 g, 11.39 mmol), N-carboxyanhydride of N-trifluoroacetyllysine (7.47 g, 27.97 mmol), and N-carboxyanhydride of L-tyrosine (1.6 g, 7.73 mmol) were placed in a single-neck flask with a magnetic stirrer. This mixture was dissolved by adding dry dioxane (289 mL). Distilled diethylamine (60 μL) was added. The resulting mixture was stirred mechanically for 24 hours at room temperature. Acetone (116 mL) was added to the mixture and the solution was slowly poured into a mixture of acetone (173 mL) and water (578 mL). The suspension was stirred and filtered. The solid was dried under vacuum at NMT 45° C. to give 12.02 g protected copolymer-1 (94.7% of yield).
example 2
Deprotection of Benzyl Group From poly[L-Ala, 5-benzyl-L-Glu, N6-TFA-L-Lys, L-Tyr] to poly[L-Ala, L-Glu, N6-TFA-L-Lys, L-Tyr]
[0043]12.02 g of protected copolymer-1, from Example 1, was suspended in 72 mL of 33% HBr / HOAc. The mixture was stirred at room temperature for 17 hours and the solution became clear. The mixture was extracted and washed with n-heptane (190 mL). The lower layer of the mixture was transferred into a mixture of water (240 mL) and n-heptane (120 mL). The precipitate was filtrated and dried to give trifluoroacetyl-glatiramer as a white solid.
example 3
Deprotection of Trifluoroacetyl Group From poly[L-Ala, L-Glu, N6-TFA-L-Lys, L-Tyr] to poly[L-Ala, L-Glu, L-Lys, L-Tyr]
[0044]9.5 g of trifluoroacetyl-glatiramer, from Example 2 was reacted with water (120.2 mL) and 40% tetrabutylammonium hydroxide in water (52.2 mL, 3 eq) for 24 hours at room temperature. The pH of the mixture was adjusted to 3˜4 by acetic acid (20 mL) to give a glatiramer acetate solution, and ultrafiltration was conducted by using a 3 kilodalton membrane to remove the low-molecular weight impurities. After 2 cycles of continuous water ultrafiltration, the resulting product is concentrated and lyophilized to give glatiramer acetate (Copolymer-1) as a pure white solid (4.7 g, 60% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com