Insecticide formulations
a technology of insecticide and formulation, which is applied in the field of insecticide formulation, can solve the problems of affecting affecting the survival of insects, and losing the efficacy of most insecticide formulations, especially liquid-based preparations, and achieving the effect of prolonging the effective field life of insecticid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Insecticidal Formulations
[0045]As illustrated in Table 1, formulations of insecticides, such as chlorpyrifos-methyl, may be incorporated into microcapsules by forming the capsule in the presence of an inert liquid, such as a hydrocarbon fluid and 1-nonanal. Various insecticidal formulations were prepared with different amounts of EDA in a continuous or in-line process as described herein.
[0046]
TABLE 1Example Insecticidal Capsule FormulationsFormulation12811Chlorpyrifos-methyl19.419.419.419.4SOLVESSO ™ 150 ND19.019.019.019.0hydrocarbon fluid1-nonanal0.380.380.380.38PAPI ® 270.62310.62310.62310.6231polymethylenepolyphenylisocyanateEthylenediamine (EDA)0.16040.13950.12560.0977GOHSENOL ® GL031.98861.98861.98861.9886polyvinyl alcoholGEROPON ® SDS0.24480.24480.24480.2448AVICEL ® CL 6110.110.110.110.11stabilizerKELZAN ® ASX0.020.020.020.02xanthan gumPROXEL ® GXL0.050.050.050.05preservativeAntifoam 100 IND0.07560.07560.07560.0756Deionized (DI) water34.734.734.734.7finishing w...
example 2
Evaluation of the Insecticidal Formulations
[0051]Properties of the insecticide formulations formed from the ingredients listed in Table 1 (i.e., formulations 1, 2, 8 and 11) were determined over time. The properties evaluated were pH, particle size, viscosity and syneresis. Such properties were determined immediately after formation (“Initial”). Samples of each of the formulations 1, 2, 8 and 11 were stored at 54° C., 40° C., freezing temperature (FT, about 0° C.) and room temperature (RT, about 25° C.). The properties of the samples stored at 54° C., 40° C., FT and RT were determined after 2 weeks and the properties of the samples stored at 40° C. and RT were determined after 4 weeks. Tables 1 through 5 provide data showing a comparison of each of the properties of formulations 1, 2, 8 and 11 observed after storing each of the formulations as described.
[0052]The pH of the samples of formulations 1, 2, 8 and 11 stored at different temperatures for time periods of 2 weeks and 4 weeks...
example 3
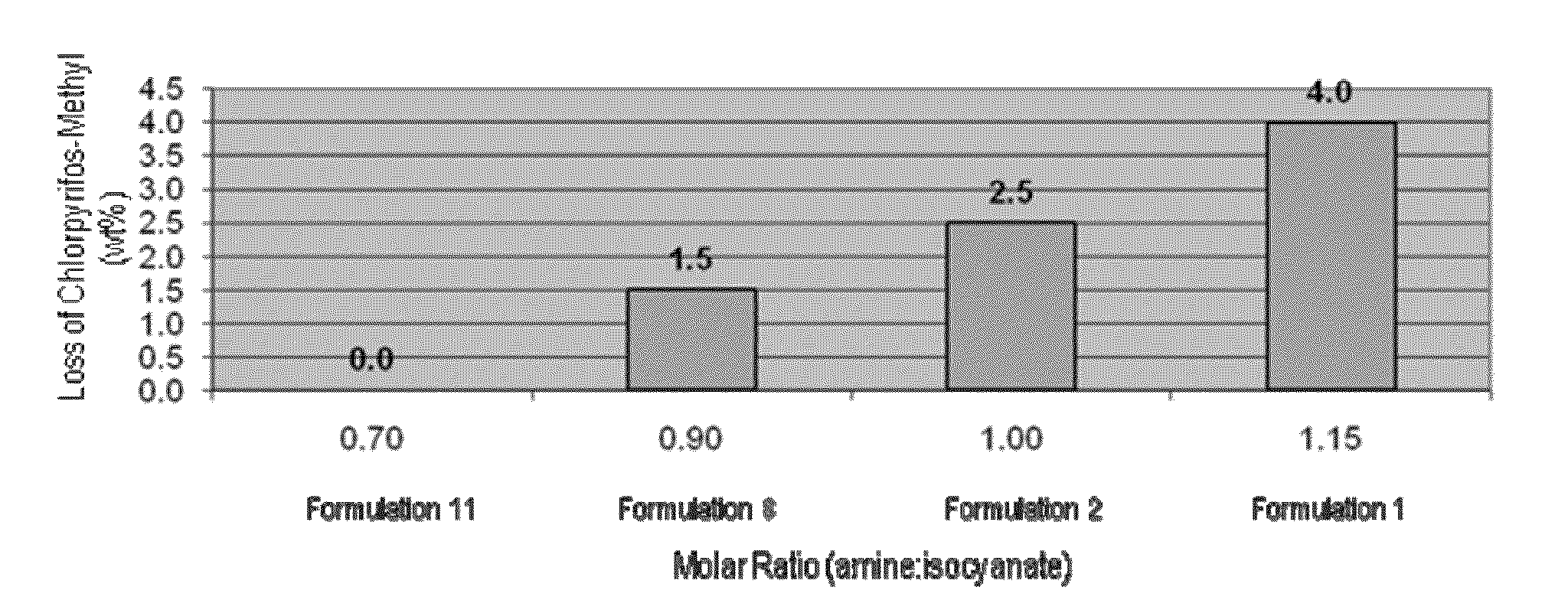
Chlorpyrifos-Methyl Degradation as a Function of Molar Ratio of Aminelsocyanate Groups in the Cross-Linking Amine and Isocyanate Monomer Emulsion Used to Prepare Insecticidal Capsule Formulations
[0061]Degradation of chlorpyrifos-methyl as a function of the molar ratio of amine:isocyanate groups in the cross-linking amine (EDA) and isocyanate monomer (PAPI™ 27) emulsion used to prepare the insecticidal capsule formulations was determined. In each of formulations 1, 2, 8 and 11, an amount of chlorpyrifos-methyl (in wt %) was determined in the samples stored at 54° C., 40° C., freezing temperature (FT, about 0° C.), and room temperature (RT, about 25° C.). The amount of the chlorpyrifos-methyl in each of the formulations was determined immediately after formation of the sample (“Initial”). Additionally, the amount of the chlorpyrifos-methyl in the samples stored at 54° C., 40° C., FT and RT was determined after 2 weeks and the amount of the chlorpyrifos-methyl in the samples stored at ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com