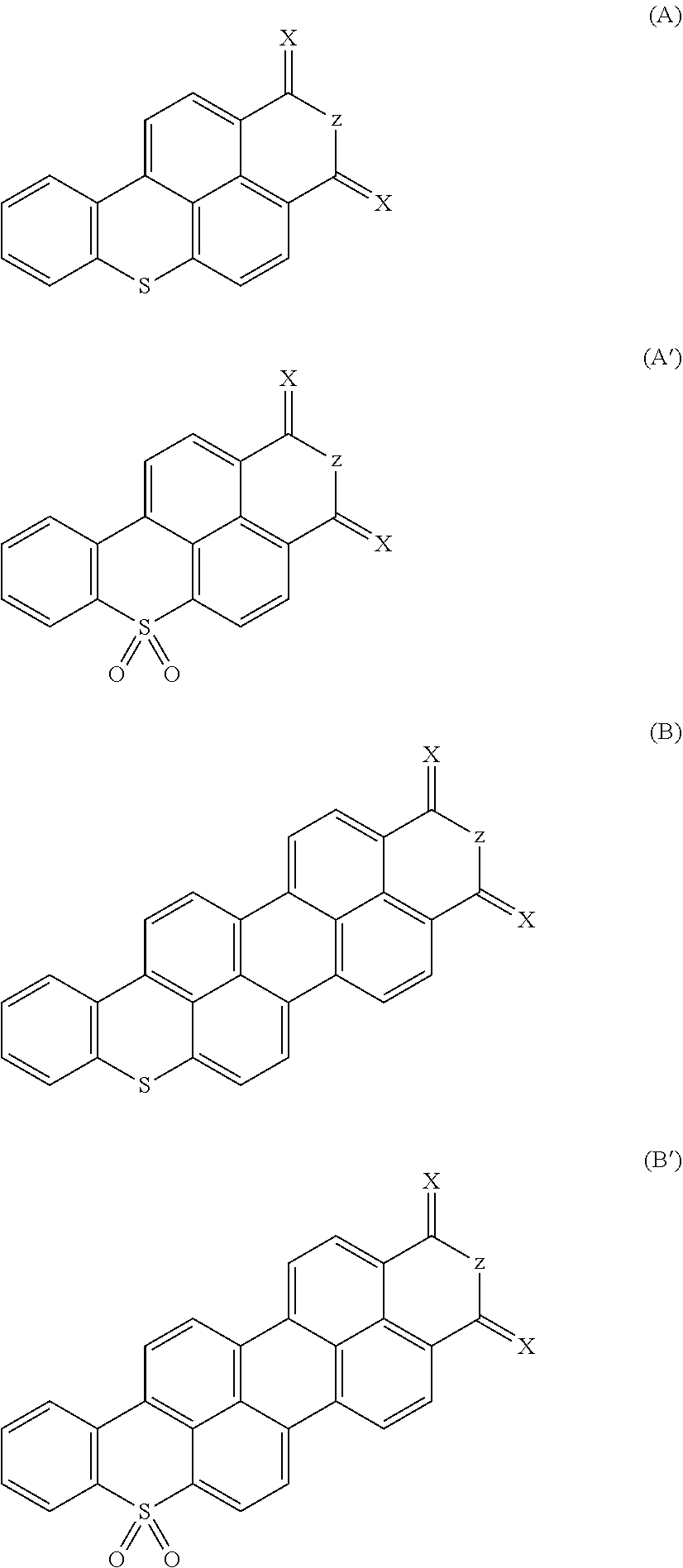
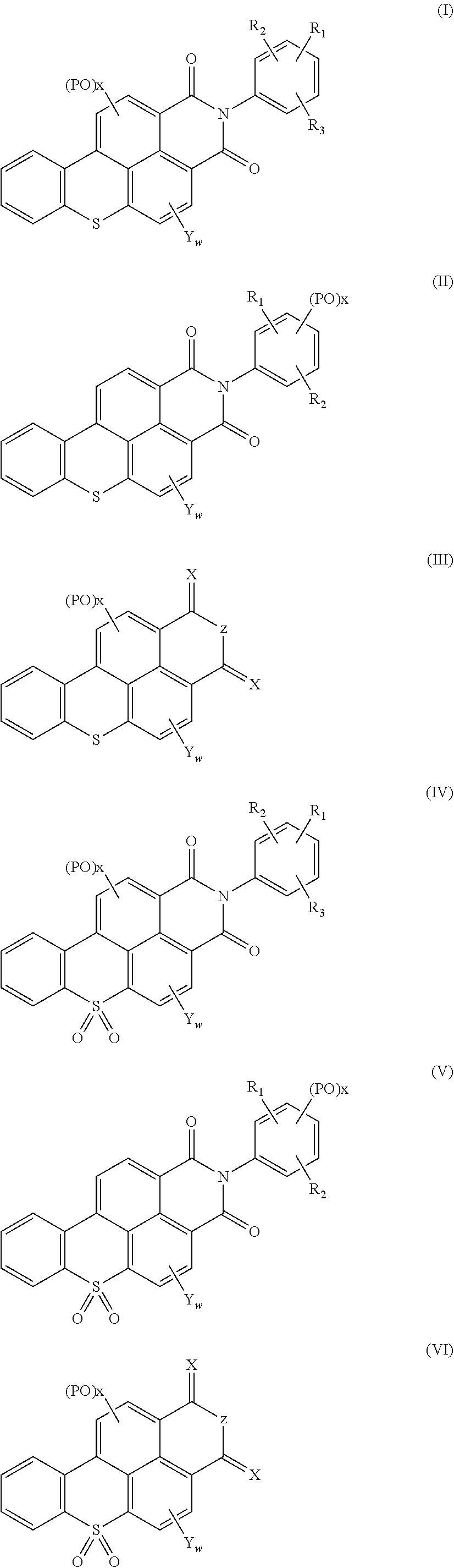
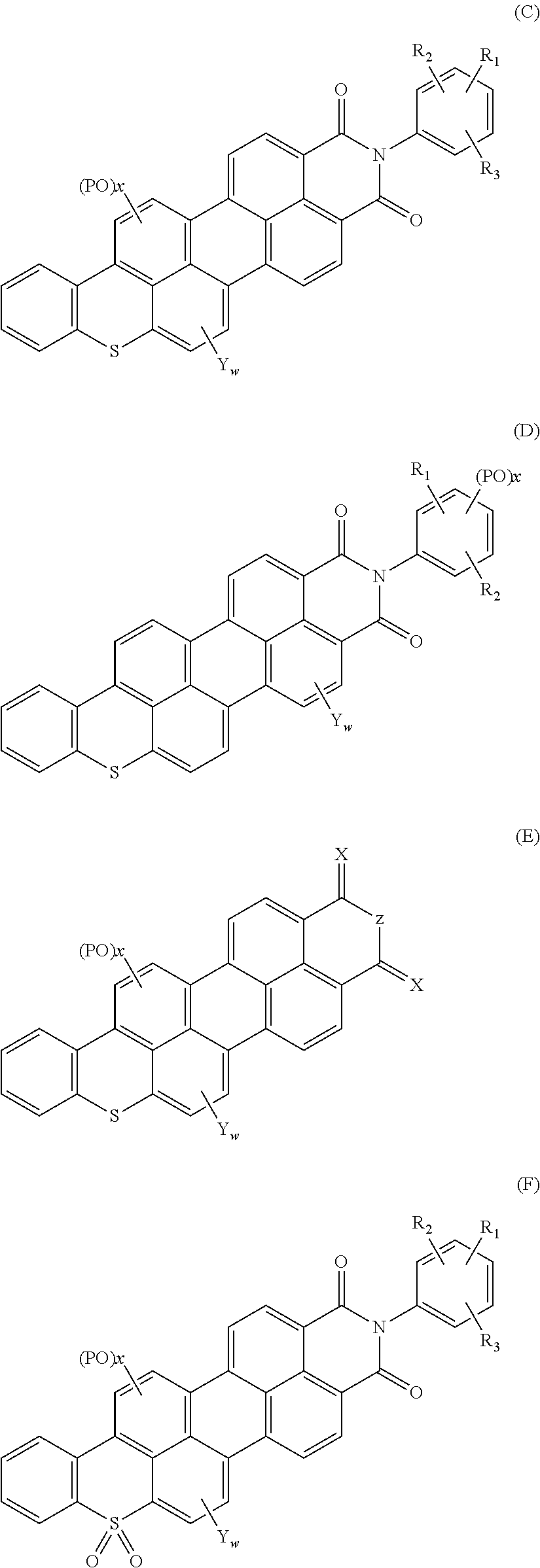
Polycyclic aromatic hydrocarbon compounds containing an S atom or S(═O)2 group in their basic structure
a technology of polycyclic aromatic hydrocarbons and aromatic hydrocarbons, applied in the direction of identification means, naphthalimide/phthalimide dyes, instruments, etc., can solve the problems of counterfeit or diverted products easily introduced into the supply chain, counterfeit or diverted documents are increasingly counterfeited around the world, and it is even more difficult to distinguish between fake documents and originals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Preparation of 4,4′,4″-((2-(2,6-diisopropylphenyl)-1,3-dioxo-2,3-dihydro-1H-thioxantheno[2,1,9-def]isoquinoline-4,8,12-triyl)tris(oxy))tribenzenesulfonate. The orange solid of sodium 4,4′,4″-((2-(2,6-diisopropylphenyl)-1,3-dioxo-2,3-dihydro-1H-thioxantheno[2,1,9-def]isoquinoline-4,8,12-triyl)tris(oxy))tribenzenesulfonate
[0094]Compound 4,8,12-trichloro-2-(2,6-diisopropylphenyl)-1H-thioxantheno[2,1,9-def]isoquinoline-1,3(2H)-dione (0.3 g), sodium 4-hydroxybenzenesulfonate (0.41 g) and anhydrous potassium carbonate (0.73 g) were added to 10 mL of N-methyl-2-pyrrolidinone (NMP). The solution was heated at 130° C. with good stirring within 4 hours. After boiling a further 4 hours, the solution was cooled at room temperature, after a minute the liquor was added to dichloromethane (100 ml) and the precipitate was filtered and dried at 60° C. to give a orange solid of sodium 4,4′,4″-((2-(2,6-diisopropylphenyl)-1,3-dioxo-2,3-dihydro-1H-thioxantheno[2,1,9-def]isoquinoline-4,8,12-triyl)tris(ox...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com