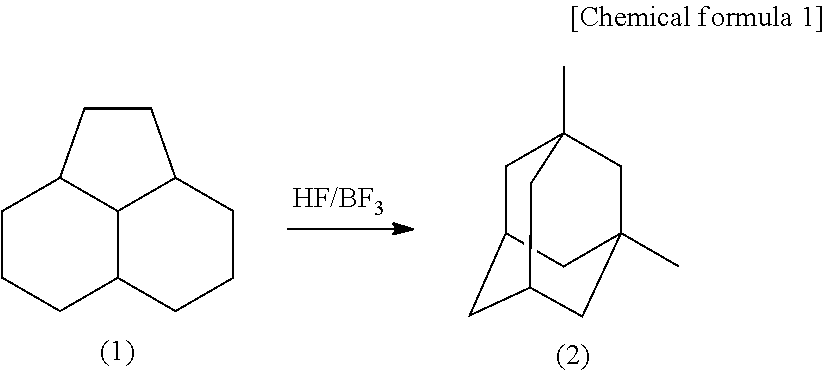
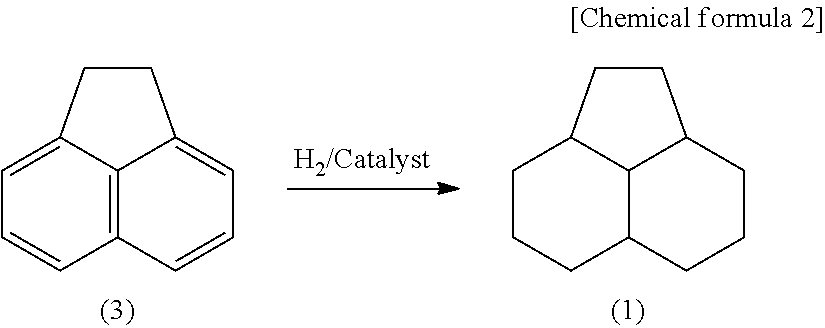
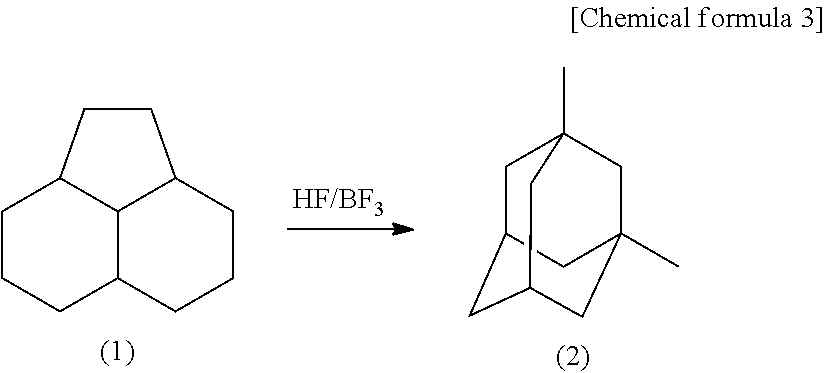
Method for producing 1,3-dimethyladamantane
a technology of dimethyladamantane and 1,3-dimethyladamantane, which is applied in the direction of organic chemistry, chemical apparatus and processes, hydrocarbon preparation catalysts, etc., can solve the problems of low yield of 38.7%, high cost of pt and re in the catalyst, and no selective production method of 1,3-dimethyladamantane at a high yield by using hf.bf/sub>catalys
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0044]PHA was subjected to an isomerization reaction by use of a Hastelloy autoclave having a capacity of 0.5 L and including an electromagnetic stirring device, a heating device, a gas and liquid supply opening and a reaction product discharge opening. 50 g (2.5 mol) of HF produced by Morita Chemical Industries Co., Ltd. and 100 g (0.6 mol) of PHA were put to a reactor, and 16 g (0.24 mol) of BF3 produced by Stella Chemifa Corporation was supplied. Then, these substances were heated to a temperature of 100° C. by the heating device with no solvent, and stirred for 4 hours while the temperature was kept at 100° C. The reaction product solution was sampled. The yield of 1,3-dimethyladamantane was 77% with respect to PHA as the material. The yield of 1-ethyladamantane as an intermediate was 15% with respect to PHA as the material, while no high boiling point compound was observed. Then, as a result of being left still, the reaction product solution was divided into two layers, namely,...
example 2
[0045]A reaction was carried out under substantially the same conditions as those in Example 1 except that HF and BF3 recovered in Example 1 were used. The yield of 1,3-dimethyladamantane was 75%, and deactivation of the catalysts was not observed. Like in Example 1, no high boiling point compound was observed.
example 3
[0046]PHA was subjected to an isomerization reaction by use of a Hastelloy autoclave having a capacity of 0.5 L and including an electromagnetic stirring device, a heating device, a gas and liquid supply opening and a reaction product discharge opening. 50 g (2.5 mol) of HF produced by Morita Chemical Industries Co., Ltd. and 100 g (0.6 mol) of PHA were put to a reactor, and 16 g (0.24 mol) of BF3 produced by Stella Chemifa Corpration was supplied. Then, these substances were heated to a temperature of 80° C. by the heating device with no solvent, and stirred for 4 hours while the temperature was kept at 80° C. The reaction product solution was sampled. The yield of 1,3-dimethyladamantane was 51% with respect to PHA as the material. The yield of 1-ethyladamantane as an intermediate was 42% with respect to PHA as the material, while no high boiling point compound was observed. Then, as a result of being left still, the reaction product solution was divided into two layers, namely, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com