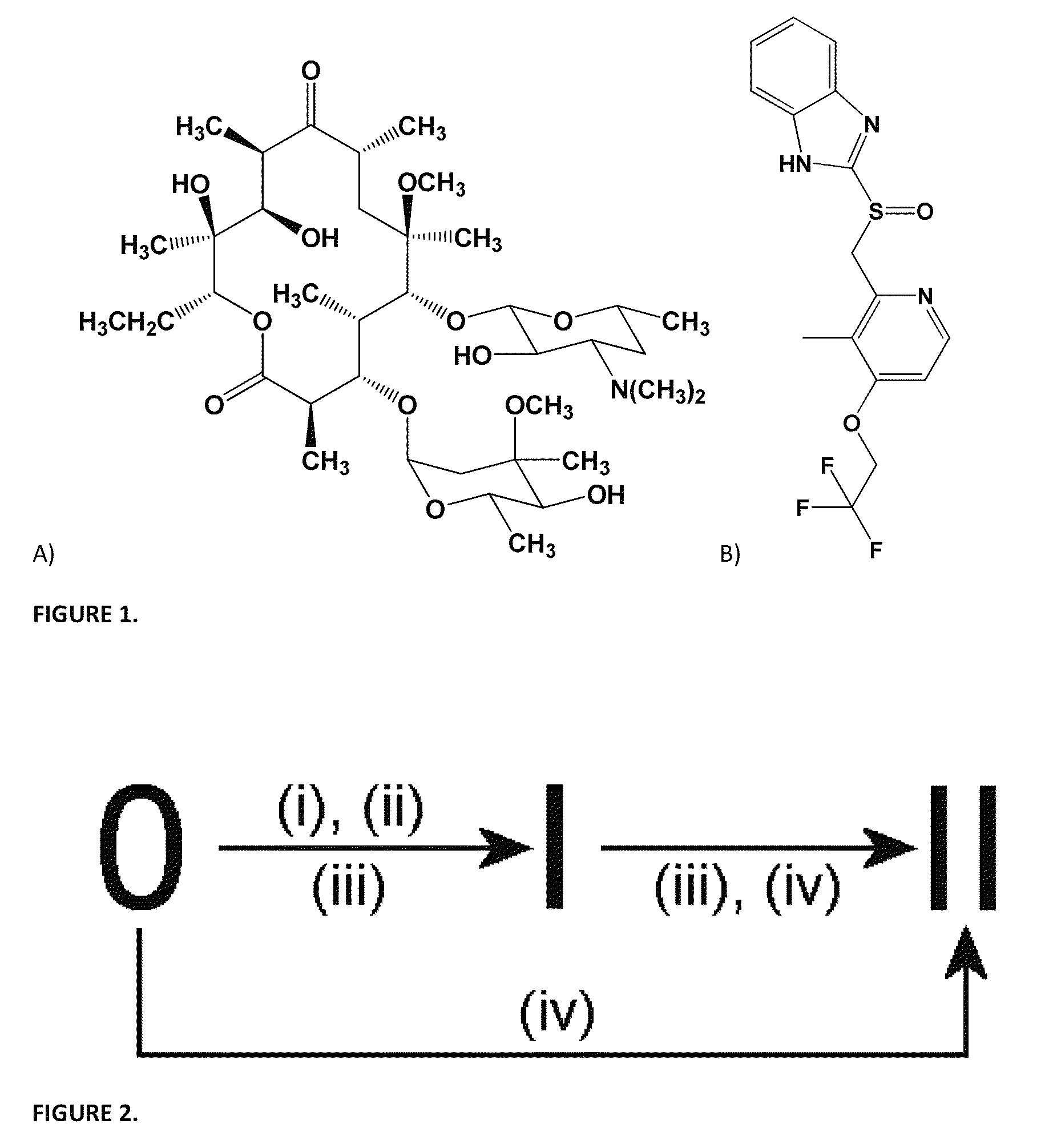
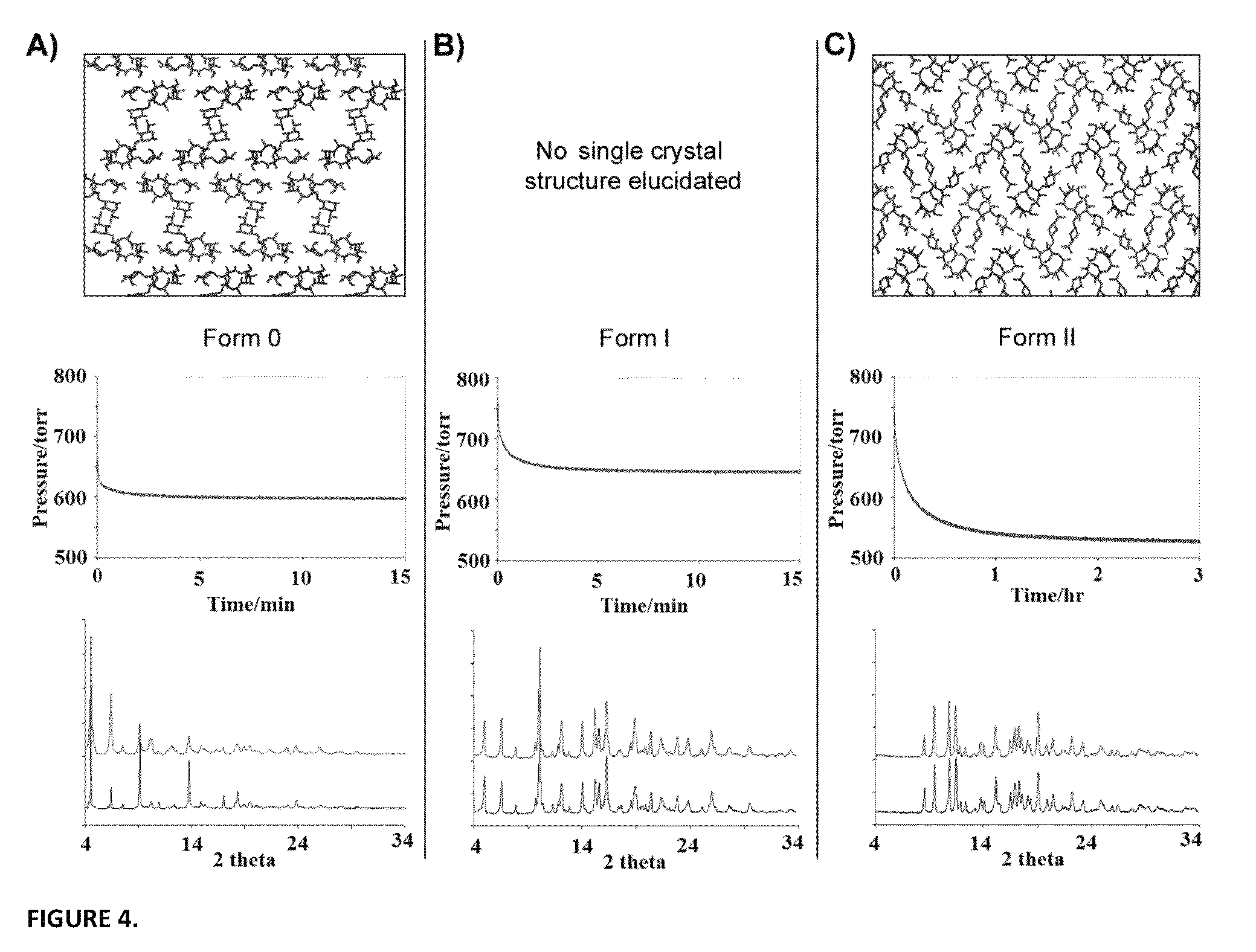
Method for transforming pharmaceutical crystal forms
a technology of pharmaceutical crystals and transformation methods, applied in the field of gas-induced transformation methods of pharmaceutical crystals, can solve the problems of difficult industrial application of techniques, poor crystalline form absorbing, and low stability and reactivity, and achieve the effect of facilitating the transformation of different polymorphic forms of organic solids and shortened durations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036]Unless otherwise defined, all technical and scientific terms used herein have the same meaning as commonly understood by one of ordinary skill in the art to which this invention belongs. All publications, patent application, patents, and other references mentioned herein are incorporated by reference in their entirety.
[0037]The invention teaches that simple pressurization of polymorphic forms of an organic solid, such as a pharmaceutical agent, can effect phase transitions among the forms with ease. More specifically, the inventive method comprises the step of subjecting an organic solid to a pressurized gas under low to moderate pressure at room temperature to a mildly elevated temperature for a period of time. The pressurized gas may be CO2, N2O, and CH4, and the pressure may start at 1 atm, whereas under slightly elevated pressure, the reaction temperature may be lowered, while the reaction time may be shortened.
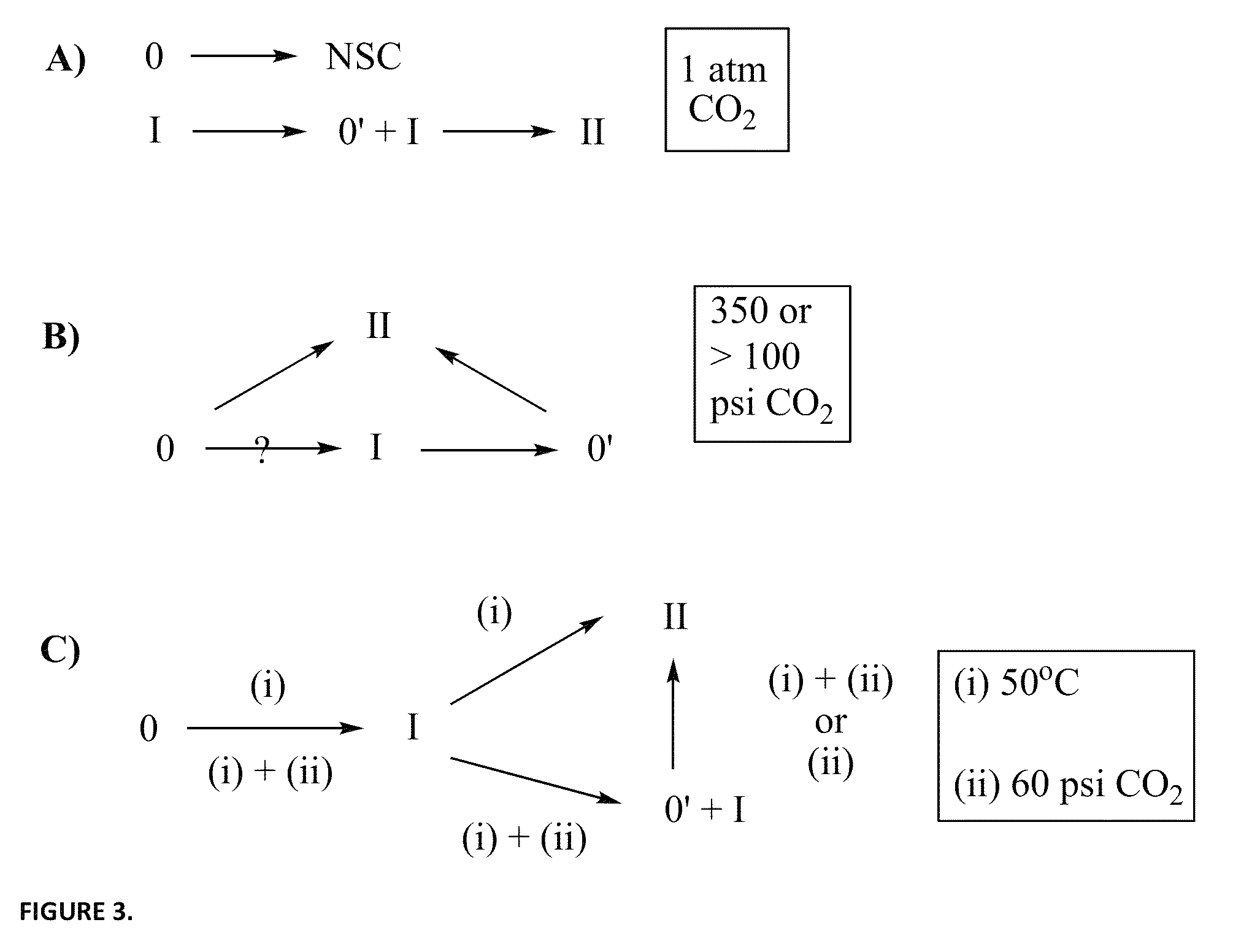
[0038]Refer to FIG. 3, which includes schematic illustrations ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com