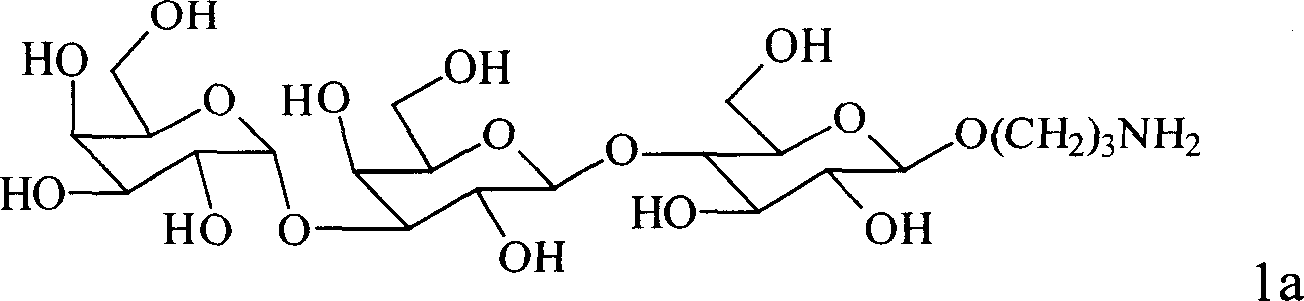
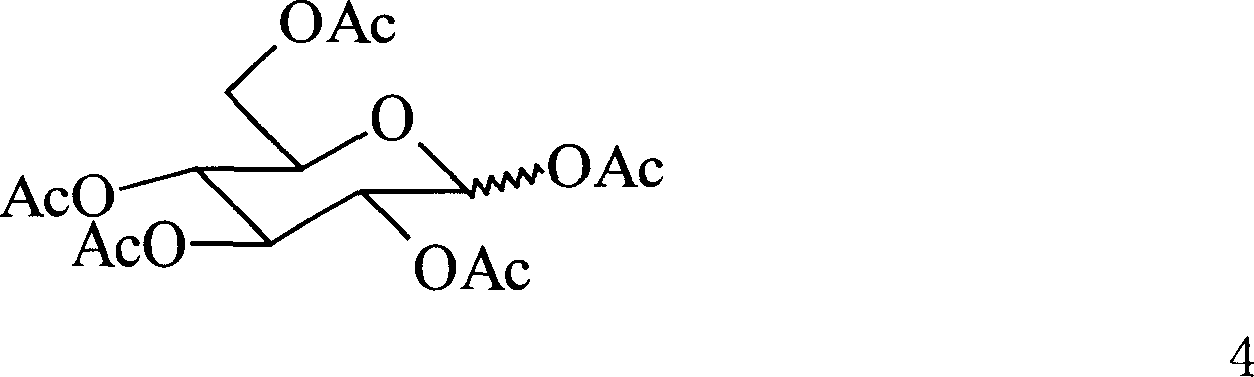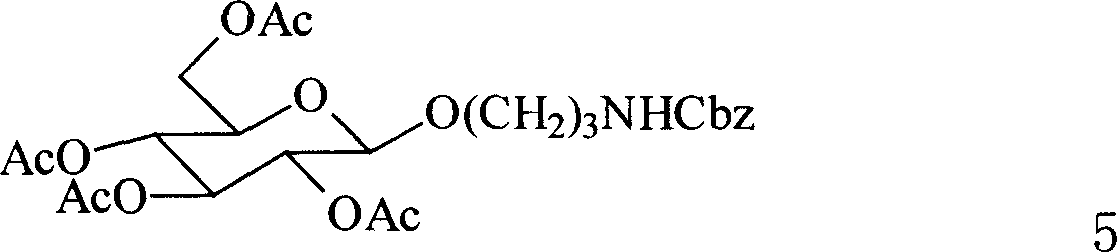New type trisaccharide and penta saccharid oligo saccharide antigen, their synthesis method and application in preparation of medicine for inhibiting exclusion reaction
A reaction and compound technology, applied in the field of novel trisaccharide and pentasaccharide oligosaccharide antigens, can solve the problems of low total yield, many synthesis steps, unsatisfactory and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2、 3
[0085] The synthetic steps of embodiment 2, trisaccharide 2:
[0086] (1) Synthesis of Compound 12
[0087] Add 4 Ȧ MS (400 mg), compound 11 (see Koeller, K.M.; Wong, C.-H. Chem. Rev. 2000, 100, 4465.) (100 mg, 0.18 mmol) and compound HO (CH) in a 50 ml reaction vial. 2 ) 3 NHCbz (48.6mg, 0.22mmol), under nitrogen protection, injected into dry CH 2 Cl 2 (10ml) was stirred for 0.5h, NIS (39.5mg, 0.18mmol), TfOH (69μl, 0.5M ether solution, 0.034mmol) were added under ice-water conditions, the reaction was completed after 2h, and triethylamine (1ml) was added to neutralize, Join CH 2 Cl 2 (15ml) was diluted, celite was filtered to remove 4 ȦMS, and the filtrate was successively washed with saturated NaHCO 3 and NaCl, dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was separated by column chromatography [eluent petroleum ether: ethyl acetate (V / V)=1:1] to obtain 56 mg of a light yellow solid, yield 49 %. 1 HNMR (500MHz, DMSO-d 6 )δ: 7.717.92(m...
Embodiment 3、 5
[0094] The synthetic steps of embodiment 3, pentasaccharide 3:
[0095] (1) Synthesis of Compound 14
[0096] Add 4 Ȧ MS (200mg), compound 9 (44.6mg, 0.069mmol), BSP (12mg, 0.063mmol) in 25ml eggplant-shaped bottle, inject dry dichloromethane (2ml), N 2 Protected, stirred for 0.5h, cooled to minus 70°C, injected with Tf 2 O (11.9μl, 19.46mg, 0.069mmol), after reacting for 10min, dissolve compound 10 (30mg, 0.063mmol) with 2ml of dry dichloromethane and add it to the reaction bottle, then slowly rise to room temperature, after 2h, TLC detection shows that the raw material After the reaction, add 2ml of triethylamine and CH 2 Cl 2 (10ml), dilute with diatomaceous earth to remove 4 ȦMS, and the filtrate is successively washed with saturated NaHCO 3 Wash with NaCl, dry over anhydrous sodium sulfate, filter, concentrate, and the residue is separated by column chromatography [eluent petroleum ether: ethyl acetate (V / V)=3:1] to obtain 56 mg of light yellow oil, the yield 90%. 1...
Embodiment 4
[0112] The preparation of embodiment 4 composition
[0113] Prepare 20 mg of the compound of general formula I, II or III of the present invention and 2 ml of physiological saline for injection into injections according to conventional injection preparation methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com