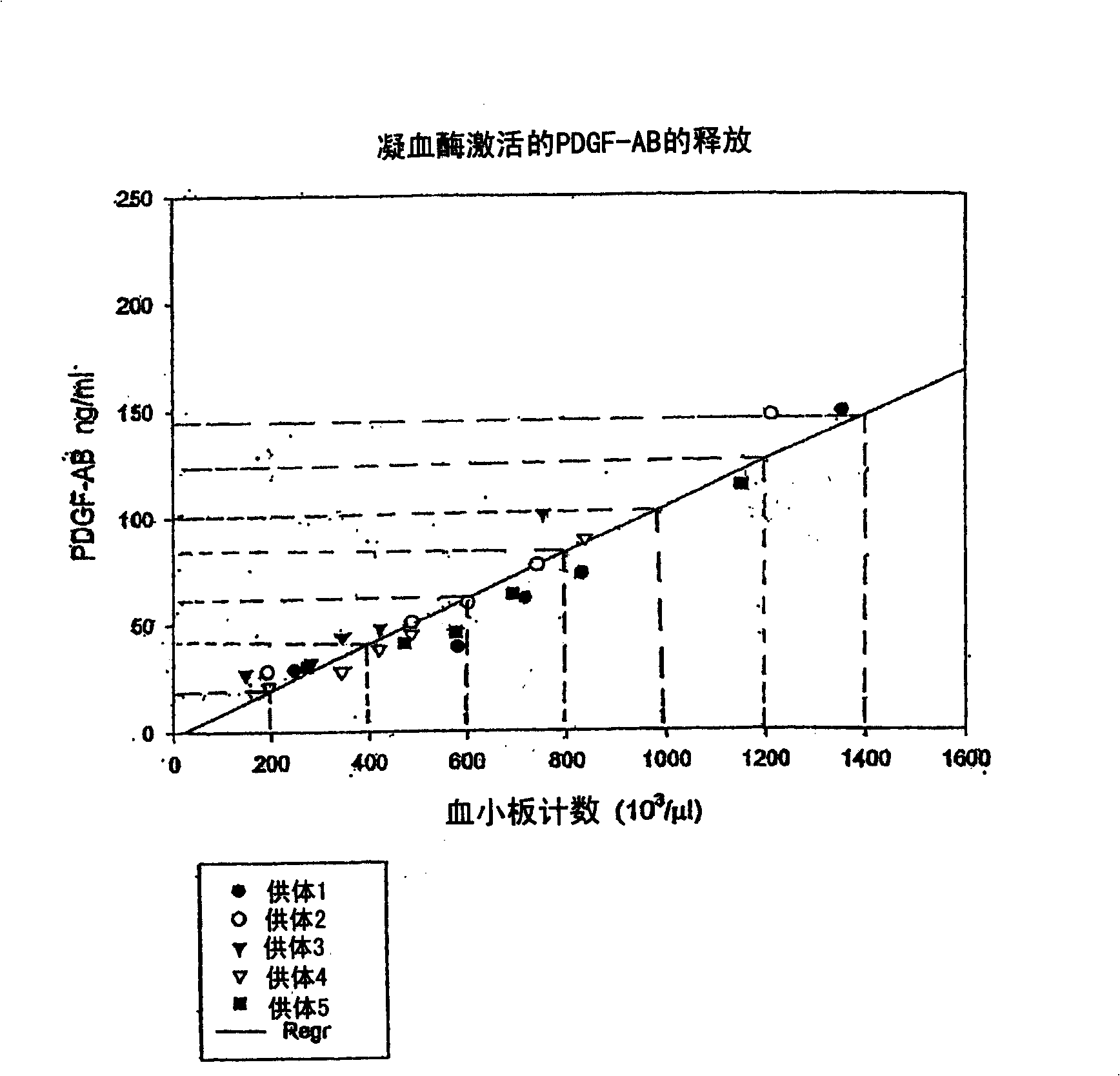
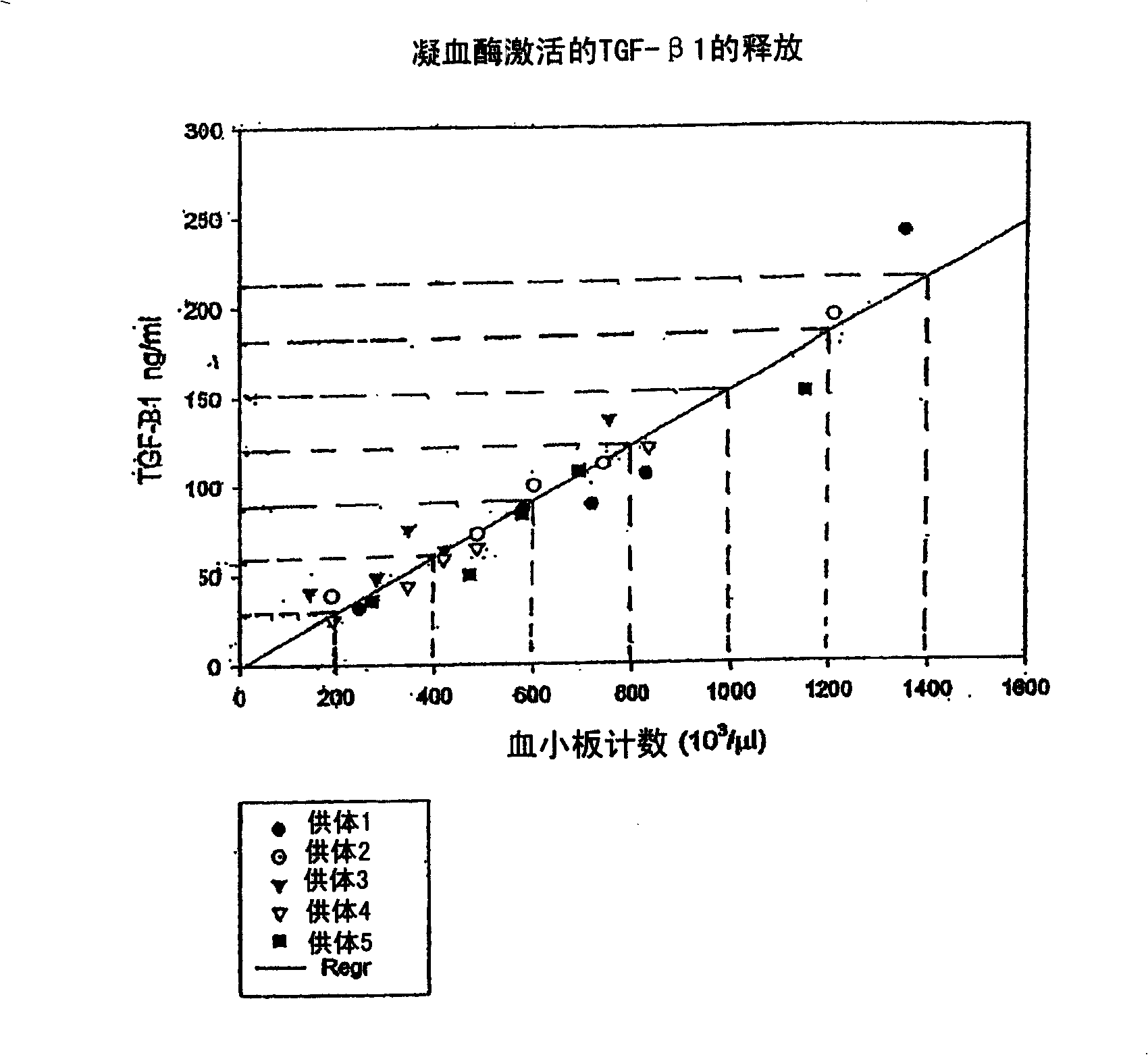
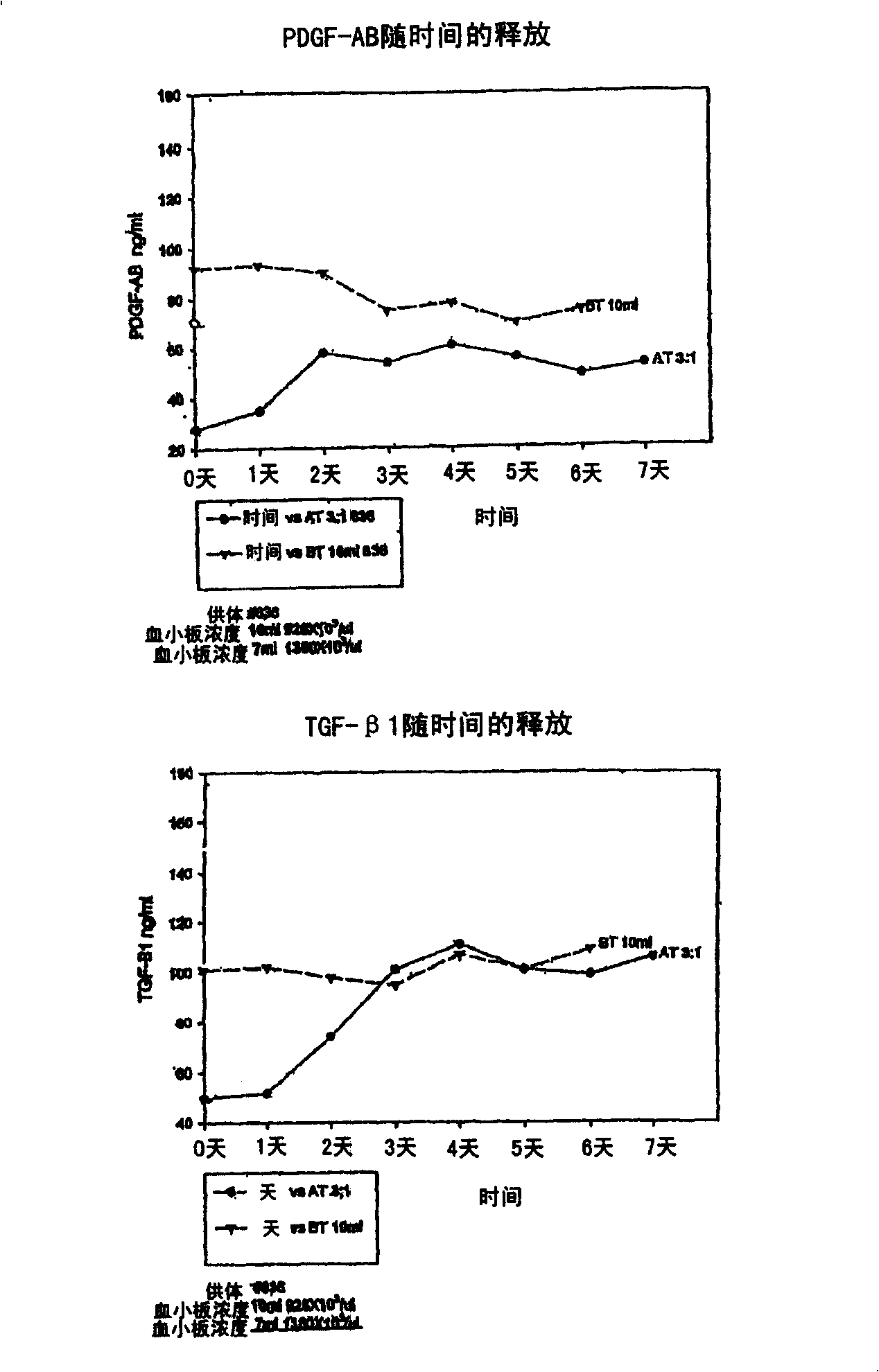
Autologous or homologous coagulant produced from anticoagulated whole blood
A coagulant and volumetric technology, applied in biocides, plant growth regulators, coagulation/fibrinolytic factors, etc., can solve the time-consuming problems of thrombin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Comparisons of relevant plasma protein levels in autologous thrombin and in whole blood samples were performed using radioimmunodiffusion (RID). Whole blood was collected in tubes containing ACD-mannitol anticoagulant. Anticoagulated whole blood was then incubated with 2 ml of 95% ethanol solution for 30 minutes. Afterwards in SMARTPREP TM System (Harvest Technologies, Plymouth, MA) centrifuged the mixture while preparing a platelet concentrate. The thrombin-containing supernatant was separated from pelleted cells and specific plasma components using a serum filtration system such as a serum filtration separator (eg, Fisher Brand, Fisher Scientific, Rochester, NY) or by aspirating the supernatant using a syringe.
[0056] Platelet poor plasma was prepared as follows. Whole blood from the same donor used to prepare autologous thrombin was collected into ACD anticoagulant solution (Cytosol Laboratories, Braintree, MA). Blood samples were centrifuged and aliquoted plas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com