Process for synthesizing phenol and acetone
一种苯酚、丙酮的技术,应用在合成苯酚及丙酮领域,能够解决分解反应不利、干扰官能度、限制等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
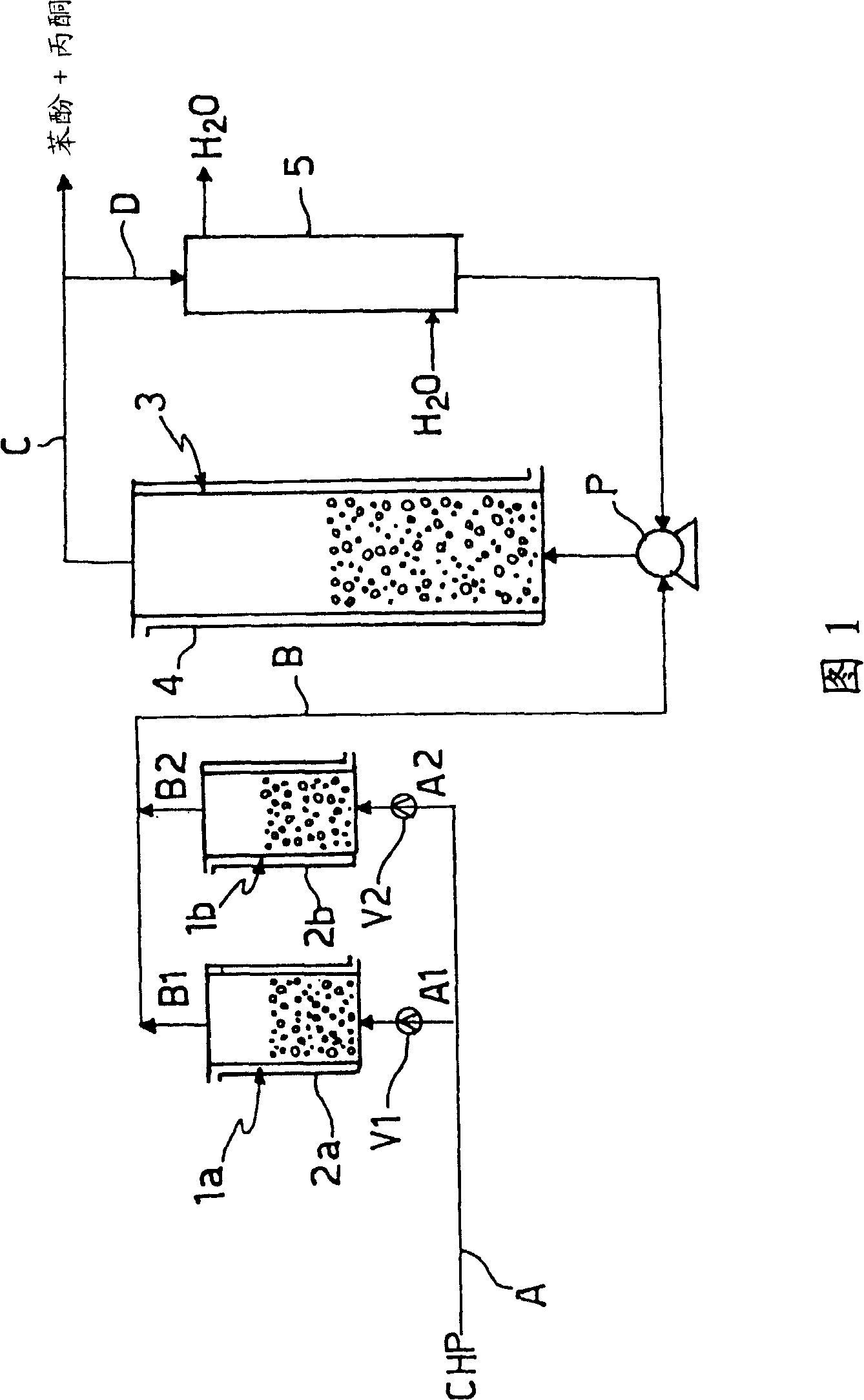
[0036] This embodiment describes a method implemented in the device shown in FIG. 1 .
[0037] Cumene hydroperoxide (CHP) with a purity of 93% and 16.4 mg of sodium cation per kg of CHP was introduced into a tank containing 600 kg of Amberlyst at a flow rate of 6 tons / hour. TM 18 ion exchange resin pretreatment reactor 1a. The reactor temperature was maintained below 20°C by a cooling jacket.
[0038]The CHP present in the pretreatment reactor 1a was analyzed by atomic absorption and no trace of sodium cations could be detected. Then the CHP, after having been mixed with 54 t / h of recycled decomposition products at a temperature of about 40°C, eg below the specified temperature, is transported along line B to the Amberlyst containing 6 t / h. TM 18 in the decomposition reactor 3.
[0039] The decomposition reactor 3 is suitably cooled by means of a cooling jacket 4 such that the temperature of the reaction products present in this reactor is approximately 42°C, although the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com