Application of yohimbine and berberine mixture in medicament preparation
A technology of berberine and yohimbine, which is applied in the application field of mixing yohimbine and berberine in pharmaceuticals, can solve the problems of reducing mortality, lack of prevention and treatment of enterogenic endotoxemia, etc., to improve survival rate, the effect of a wide range of application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] To observe the effect of combined application of berberine and yohimbine on the mortality of endotoxemia mice.
[0018] Healthy male BALB / C mice (body weight 20-25g) were randomly divided into 6 groups: the following treatments were performed respectively:
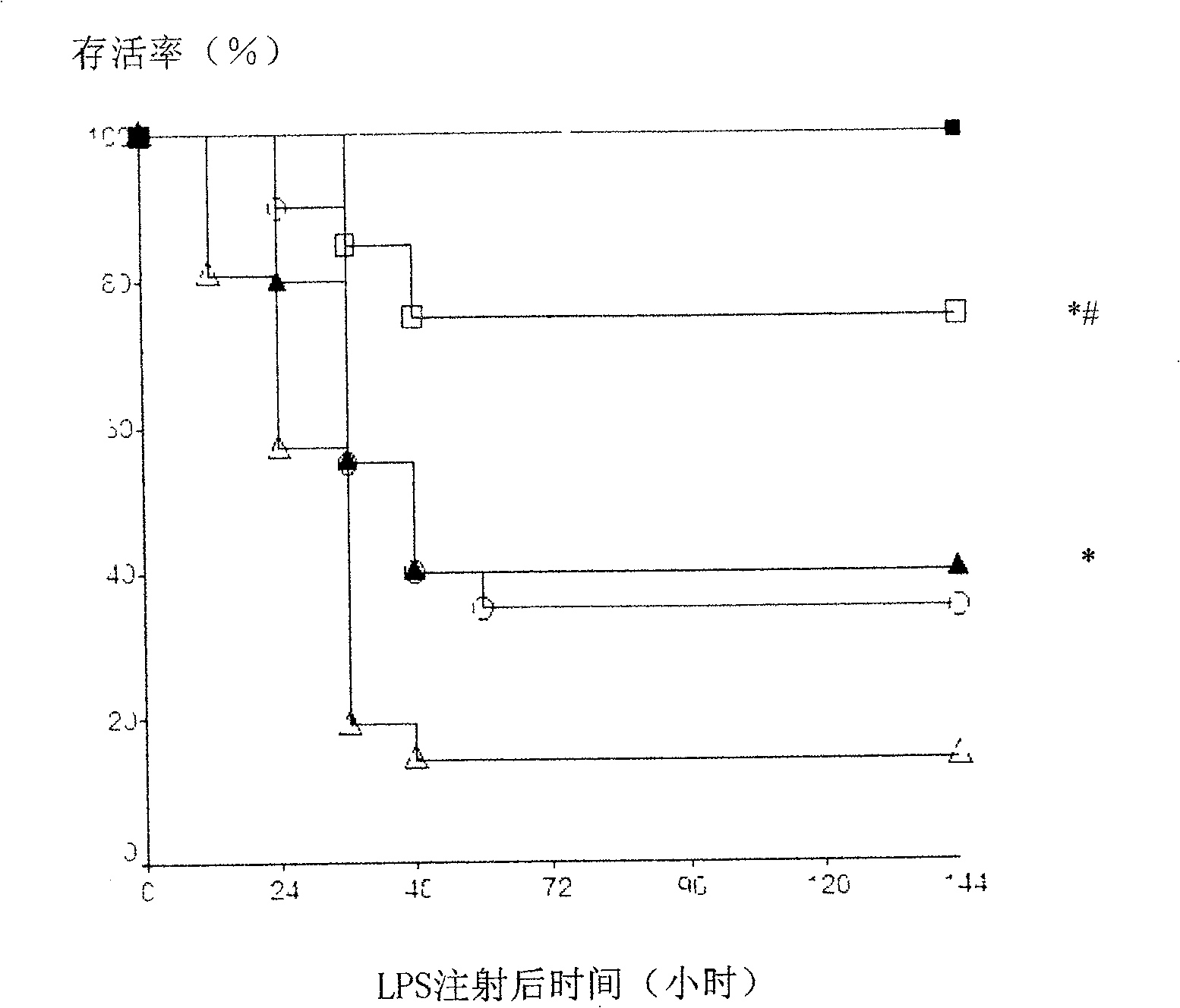
[0019] ① control group (control), figure 1 Indicated by a solid square: 3 days in advance, intraperitoneal injection of physiological saline (0.05ml / 10g), 30 minutes later intragastric administration of water (0.2ml / 10g), once a day, 1 hour after intragastric administration on the last day, intraperitoneal injection of physiological brine;
[0020] ②LPS group (LPS), figure 1 Indicated by a hollow triangle: 3 days in advance, intraperitoneal injection of normal saline (0.05ml / 10g), 30 minutes later intragastric administration of water (0.2ml / 10g), once a day, 1 hour after intragastric administration on the last day, intraperitoneal injection Toxin (LPS, 055:B5, Sigma, 20mg / kg);
[0021] ③Berberine control group [...
Embodiment 2
[0029] Effects of different doses of yohimbine combined with 50 mg / kg berberine on mortality in endotoxemic mice.
[0030] Healthy male BALB / C mice (body weight 20-25g) were randomly divided into 5 groups: the following treatments were performed respectively:
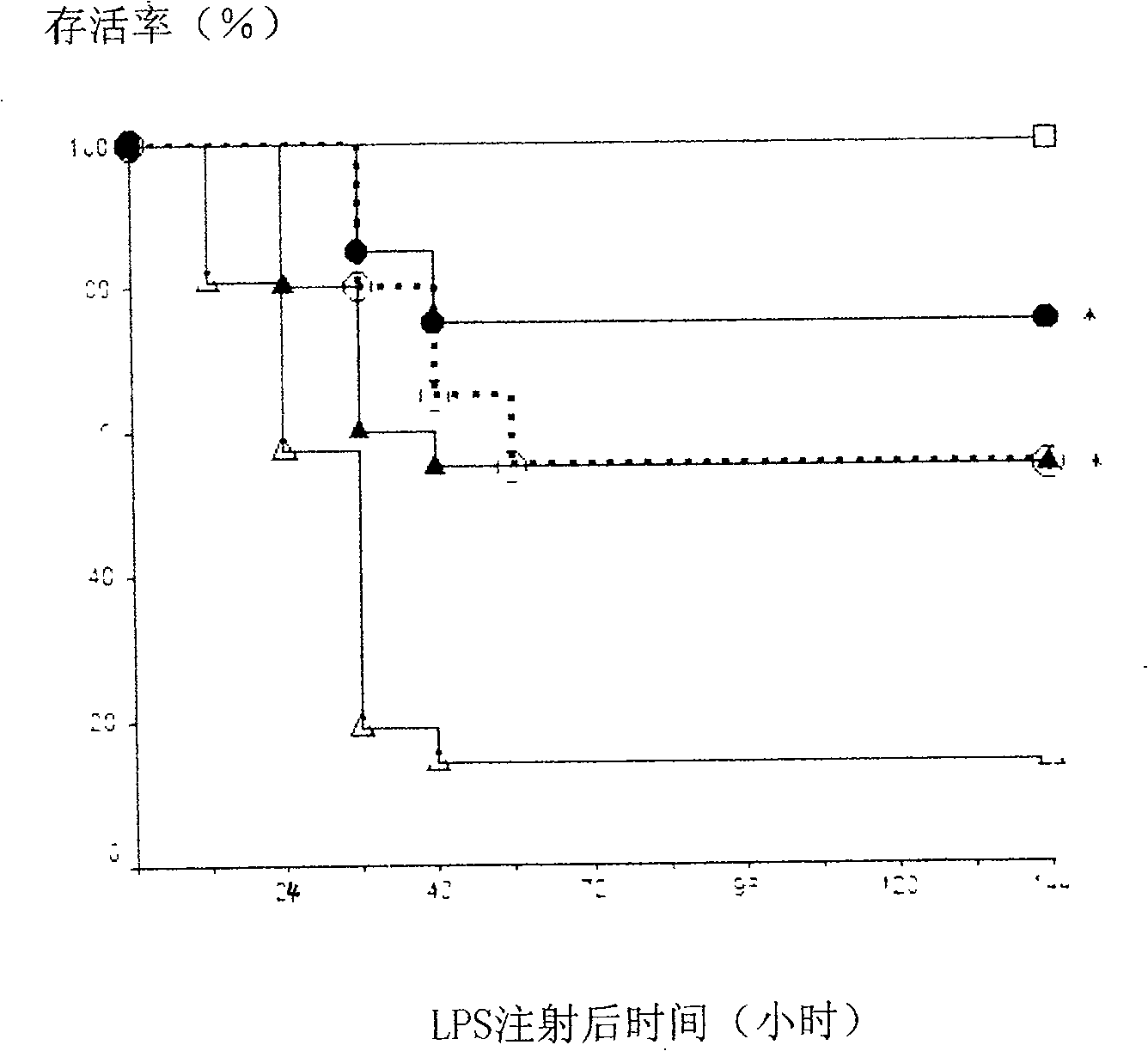
[0031] ① control group (control), figure 2 Indicated by a hollow square: 3 days in advance, intraperitoneal injection of physiological saline (0.05ml / 10g), 30 minutes later intragastric administration of water (0.2ml / 10g), once a day, 1 hour after intragastric administration on the last day, intraperitoneal injection of physiological brine;
[0032] ②LPS group (LPS), figure 2 Indicated by a hollow triangle: 3 days in advance, intraperitoneal injection of normal saline (0.05ml / 10g), 30 minutes later intragastric administration of water (0.2ml / 10g), once a day, 1 hour after intragastric administration on the last day, intraperitoneal injection Toxin (LPS, 055:B5, Sigma, 20mg / kg);
[0033] ③ 1mg / kg yohimbine and 50mg...
Embodiment 3
[0040] Effect of 2mg / kg yohimbine and 50mg / kg berberine mixed preparation (berberine and yohimbine mixture) administered orally on the mortality of endotoxemia mice.
[0041] Healthy male BALB / C mice (body weight 20-25g) were randomly divided into 4 groups: the following treatments were performed respectively:
[0042] ① Control group (control): gavage with water (0.2ml / 10g) for 3 days, once a day, and inject normal saline into the abdominal cavity 1 hour after gavage on the last day;
[0043] ②LPS group (LPS): gavage with water (0.2ml / 10g) for 3 days, once a day, and intraperitoneally inject endotoxin (LPS, 055:B5, Sigma, 20mg / kg) 1 hour after gavage on the last day;
[0044] ③ 2mg / kg yohimbine and 50mg / kg berberine mixed preparation for 3 days prevention group [berberine and yohimbine mixture (3) group]: 2mg / kg yohimbine and 50mg / kg berberine mixed preparation Stomach, once a day, for 3 days in total, 1 hour after gavage on the third day, intraperitoneally inject LPS (20mg / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com