Yellow active dye composition and its use
A technology of reactive dyes and compositions, applied in organic dyes, dyeing methods, textiles and papermaking, etc., can solve problems affecting industrial application practices, etc., and achieve good washable performance, bright shade, and good fastness effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of the yellow reactive dye composition of the present invention comprises mixing the above-mentioned dye I: dye II in a prescribed weight ratio. Mixing can be performed by various conventional methods, such as mechanical mixing methods. When mixed, Dye I and Dye II may be present in powder form, or in granular form, or in aqueous solution.
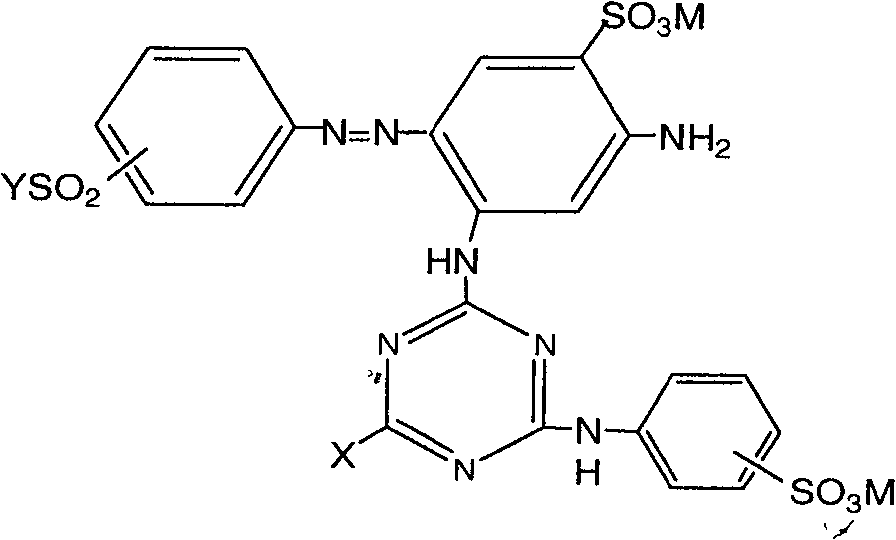
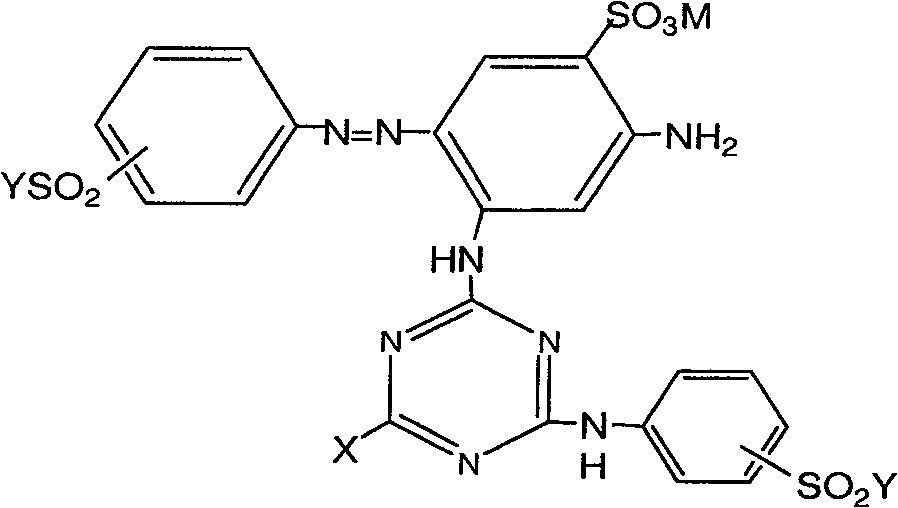
[0041] In the structural formula of dye I or II, when X is halogen, especially Cl, F, it can be prepared according to methods known in the art. Company] and CN 1443219 (2003) [DyStar Corporation] (incorporated herein by reference).
[0042] When X is -HNCN in the structural formula of dye I, it can be prepared according to methods known in the art, and its preparation method includes:
[0043] a. The 2,4-diaminobenzenesulfonic acid aqueous solution is added to the cyanuric halide suspension to carry out the first condensation. The temperature is controlled at 0-5° C., the pH value of the reaction solution i...
preparation Embodiment 1
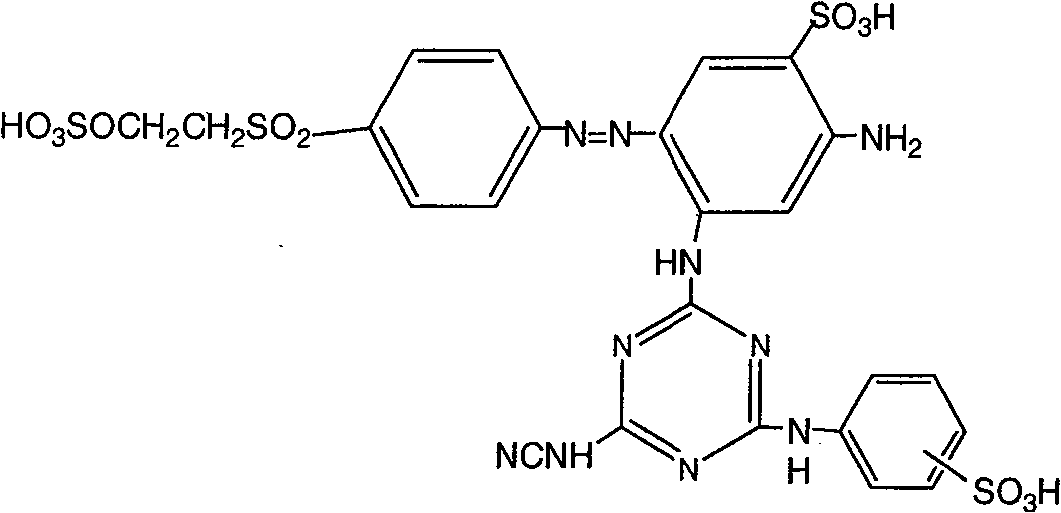
[0051] Dye I represented by the formula 1 preparation
[0052]
[0053] In a 2-liter three-necked round-bottomed flask equipped with a mechanical stirrer, a dropping funnel and a thermometer, slowly add 94 grams of 2,4-diaminobenzenesulfonic acid aqueous solution to a mixture of 92 grams of cyanuric chloride suspended in 800 milliliters of water. The suspension undergoes a first condensation. The temperature is controlled at 0 to 5° C., the pH value of the reaction solution is controlled to be 2 to 3 with sodium hydroxide solution, and the reaction time is about 2 hours.
[0054] Then, in the case of stirring, add 86.5 grams of p-aminobenzenesulfonic acid aqueous solution to the reaction solution to carry out the second condensation, the pH value of the reaction solution is controlled at 2~3, the reaction temperature rises to 50~60 ℃, and the reaction continues for about 2 to 3 hours.
[0055] Thereafter, 21 grams of cyanamide was added to the above reaction solution for...
preparation Embodiment 2
[0059] Dye II represented by the formula 1 preparation
[0060]
[0061] In a 2-liter three-necked round-bottomed flask equipped with a mechanical stirrer, dropping funnel and thermometer, 21 g of cyanamide was added to a suspension of 92 g of cyanuric chloride suspended in 800 ml of water for the first condensation. The temperature is controlled at 0-5 DEG C, the pH value of the reaction solution is controlled to be 2-3 with sodium hydroxide solution, and the reaction time is about 1 hour.
[0062] Then, the pH value of the reaction solution was adjusted to 2.0-3.0, and 94 grams of 2,4-diaminobenzenesulfonic acid aqueous solution was slowly added to carry out the second condensation. The reaction temperature is controlled at 35-40 DEG C, the pH value of the reaction solution is controlled at 2.0-3.0, and the reaction is carried out for about 4 hours.
[0063] After that, 140 grams of (β-sulfate ethyl sulfone) aniline was slowly added to the condensation solution for the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com