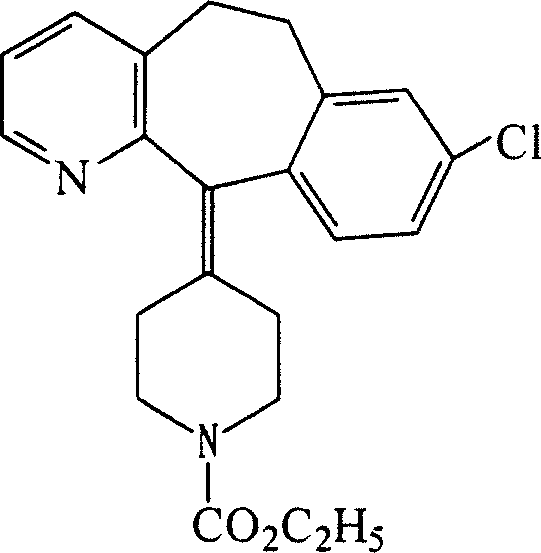
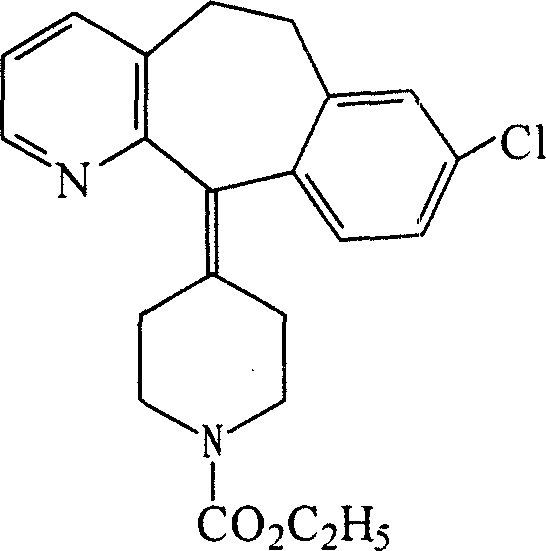
Oral cavity quick dissolved film containing civeran, and method for preparing the same
An oral instant film, loratadine technology, which is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of fragility, no improvement of defects, and unfavorable quantitative dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Determination of the dissolution time limit of loratadine oral instant film:
[0039] Take 10 sheets of medicine film randomly, one piece at a time, clamp one side of the medicine film with a clip with a jaw of about 2cm, and immerse the clip together in a water bath at 37°C, and use a stopwatch to time the medicine film from being immersed in water to dissolving And the time of breaking away from the clip is the melting time limit.
Embodiment 2
[0041] Loratadine 100g
[0042] Hydroxypropyl methylcellulose (4000 centipoise) 100g
[0043] Purified water 1000g
[0044] First, add the above-mentioned amount of hydroxypropyl methylcellulose (4000 centipoise) into the aqueous solution under stirring, fully stir and disperse to obtain hydroxypropyl methylcellulose gel, then add loratadine and stir evenly to obtain Loratadine / hydroxypropyl methylcellulose gel, degassed, spread the loratadine / hydroxypropyl methylcellulose gel on a flat plate, the coating is difficult and uneven, 70-80 ℃ blowing heating and drying, cutting to obtain loratadine oral instant film, which is white, poor in toughness, brittle, slow in melting and dispersing at the entrance, poor in mouthfeel, and sticky.
Embodiment 3
[0046] Loratadine 100g
[0047] Hydroxypropyl methylcellulose (15000 centipoise) 25g
[0048]Purified water 1000g
[0049] First, add the above-mentioned amount of hydroxypropyl methylcellulose (15000 centipoise) into the aqueous solution under stirring, fully stir and disperse to obtain hydroxypropyl methylcellulose gel, then add loratadine and stir evenly to obtain Loratadine / hydroxypropyl methylcellulose gel, degassed, spread the loratadine / hydroxypropyl methylcellulose gel on a flat plate, the coating is difficult and uneven, 70-80 ℃ blast heating and drying, cutting to obtain loratadine oral instant film, which is white, poor in toughness, brittle, slow in melting and dispersing at the entrance, and poor in mouthfeel.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com