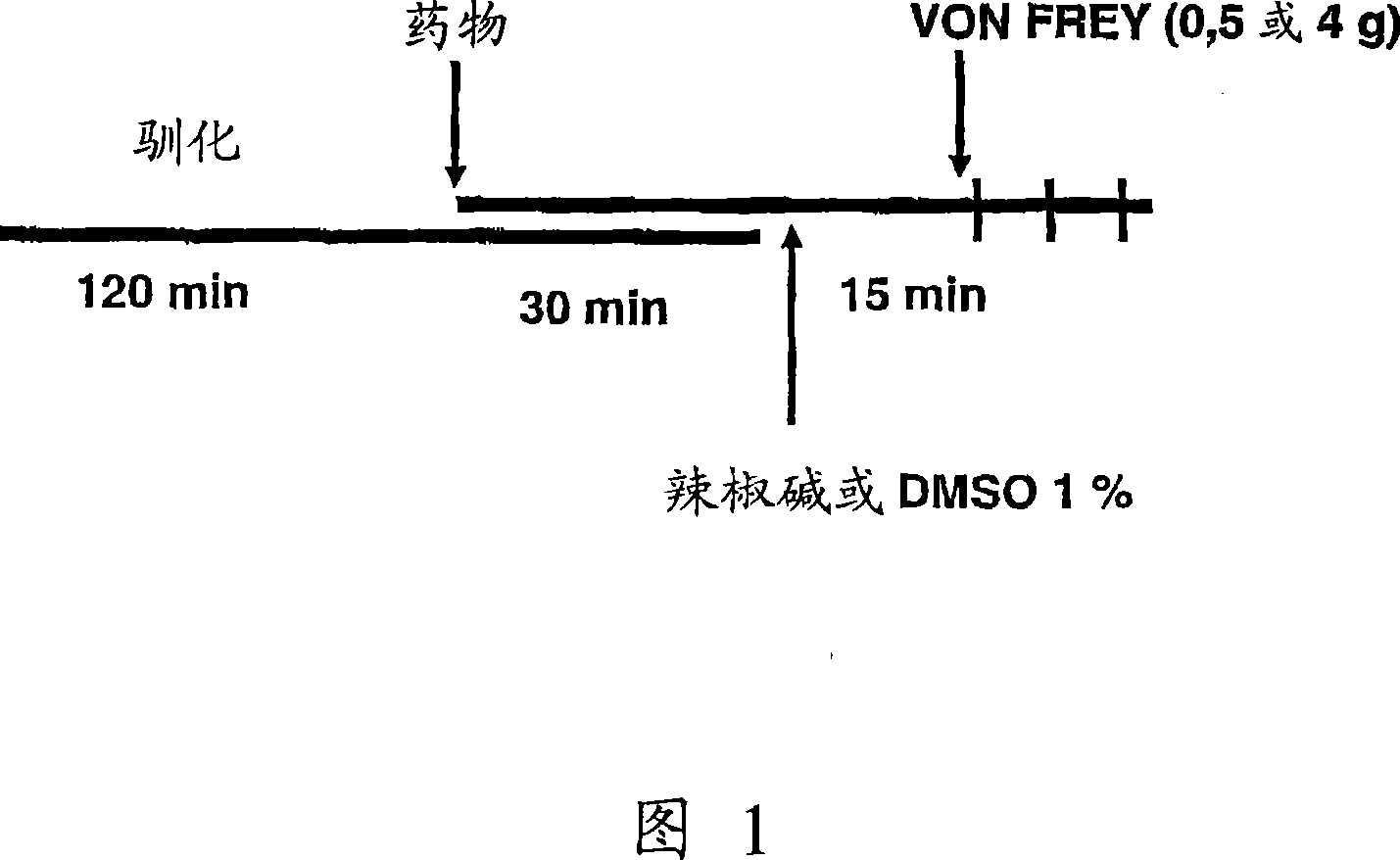
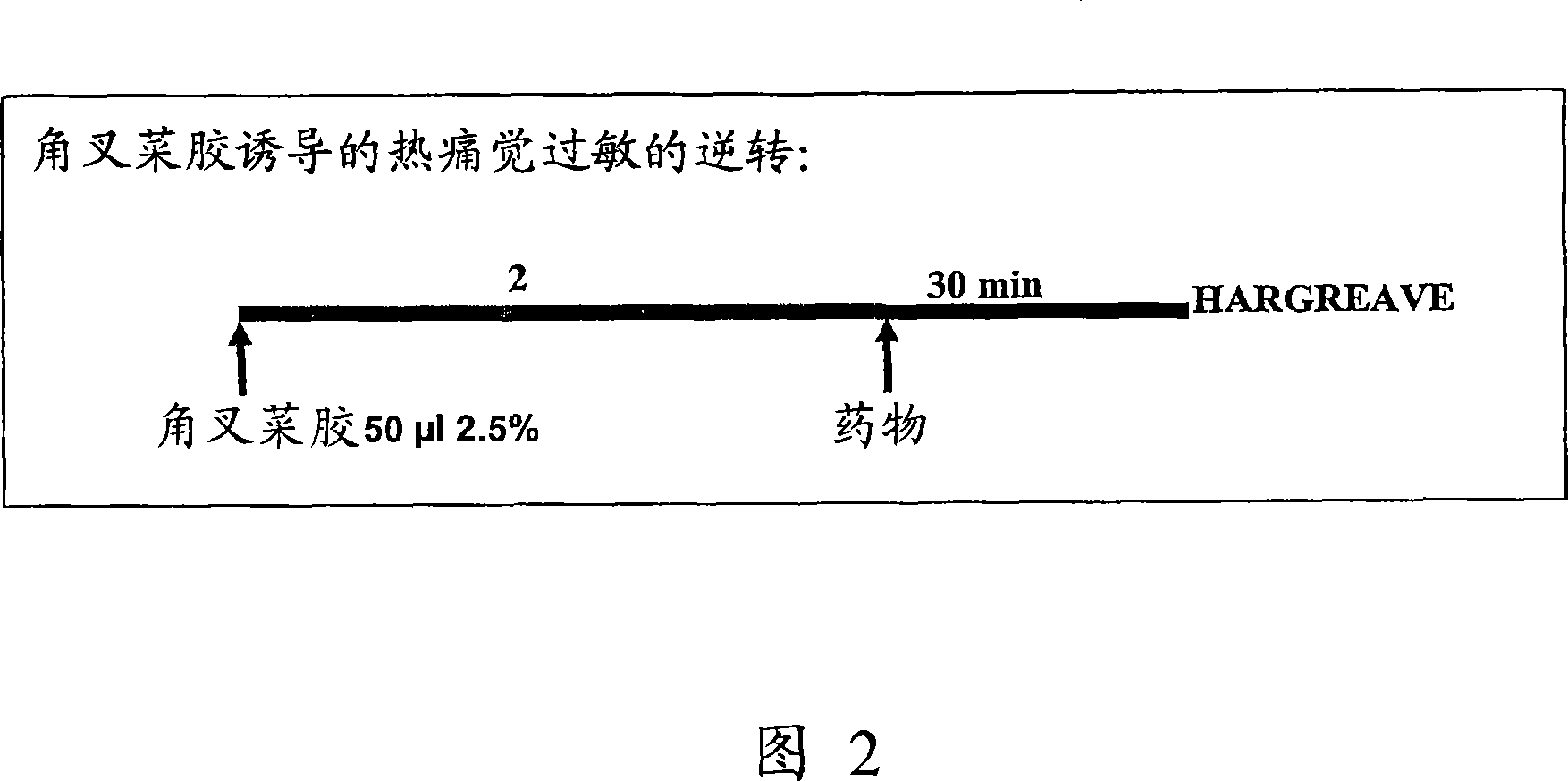
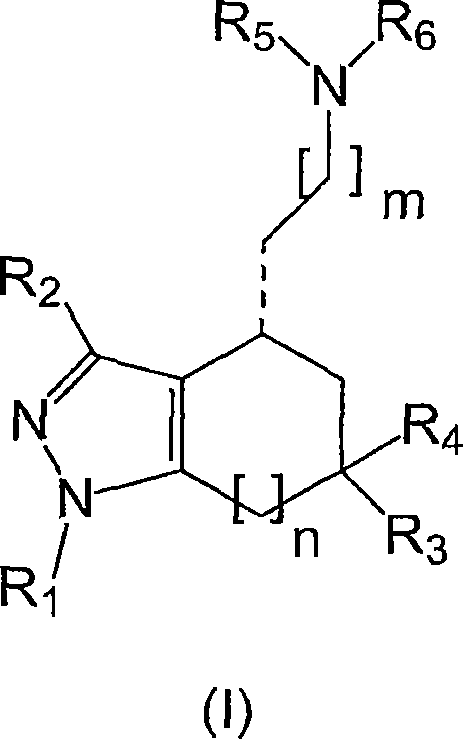
Sigma receptor inhibitors
A technology of isomers and solvates, applied in the direction of anti-inflammatory agents, non-central analgesics, medical preparations containing active ingredients, etc., can solve the problems of no given or implied effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Synthesis of 4,5,6,7-tetrahydro-4-(2-(morpholin-4-yl)ethyl)-1-phenyl-1H-indazole (1)
[0230] Step 1: Synthesis of ethyl 2-(4,5,6,7-tetrahydro-1-phenyl-1H-indazol-4-yl)acetate
[0231]
[0232] To (1,5,6,7-tetrahydro-1-phenyl-4H-indazol-4-ylidene)acetate E / Z isomer mixture (1,6g, 5,67mmol) in 50mL To the solution in EtOH was added Pd-C (150 mg, 10%) and the resulting solution was stirred on a Parr hydrogenator under nitrogen atmosphere (50 psi) for 18 hours. The reaction mixture was purged with nitrogen, filtered using Celite and the solvent was evaporated under reduced pressure to give ethyl 2-(4,5,6,7-tetrahydro-1-phenyl-1H-indazol-4-yl)acetate ( 1,60 g, 5,63 mmol, 99%, oil).
[0233] 1 H RMN (300MHz, CDCl 3 ): δ1,29(t,J=7,2Hz,3H), 1,48(m,1H), 1,73(m,1H), 1,98(m,2H), 2,45(dd, J=15,4Hz, J'=8,0Hz,1H), 2,68(m,3H), 3,26(m,1H), 4,20(q,J=7,2Hz,2H), 7 , 31(m, 1H), 7, 41-7, 52(m, 5H).
[0234] Step 2: Synthesis of 2-(4,5,6,7-tetrahydro-1-phenyl-1H-indazol-4-yl)ethan...
Embodiment 2
[0247] Synthesis of 4,5,6,7-tetrahydro-4-(2-(morpholin-4-yl)ethyl)-1-phenyl-1H-indazole oxalate (2)
[0248]
[0249] To 4,5,6,7-tetrahydro-4-(2-(morpholin-4-yl)ethyl)-1-phenyl-1H-indazole (380 mg, 1,22 mmol) in 4 mL of acetone Add HO to the solution 2 CCO 2 H.2H2 O (156 mg, 1,23 mmol) to give 4,5,6,7-tetrahydro-4-(2-(morpholin-4-yl)ethyl)-1-phenyl-1H-indazole oxalate (318 mg, 0,79 mmol, 65%, white solid).
[0250] m.p.=150-151℃
[0251] 1 H RMN (300MHz, DMSO-d 6 , TFA): δ1,36(m,1H), 1,60(m,1H), 1,71-1,93(m,3H), 2,04(m,1H), 2,56-2, 77(m, 3H), 3,03(m, 2H), 3,21(m, 2H), 3,44(d, J=12,7Hz, 2H), 3,62(t, J=11, 6Hz, 2H), 3,92(m, 2H), 7,29(m, 1H), 7,38-7,49(m, 4H), 7,62(s, 1H), 9,69(m , 1H).
Embodiment 3
[0253] 1-(3,4-dichlorophenyl)-4,5,6,7-tetrahydro-4-(2-(4-phenylpiperidin-1-yl)ethyl)-1H-indazole ( 3) Synthesis of
[0254]Step 1: Synthesis of 1-(3,4-dichlorophenyl)-1,5,6,7-tetrahydro-4H-indazol-4-one
[0255]
[0256] A mixture of 1-(3,4-dichlorophenyl)hydrazine hydrochloride (6,78 g, 31,8 mmol) and anhydrous sodium acetate (2,60 g, 31,8 mmol) in n-BuOH (20 mL) was slowly added to 2 - A solution of ((dimethylamino)methylene)cyclohexane-1,3-dione (5,32 g, 31,8 mmol) in n-BuOH (100 mL) and acetic acid (5 mL). The resulting mixture was heated to reflux for 2 hours and the reaction was monitored using TLC. The solvent was evaporated under reduced pressure, the residue was diluted with AcOEt and washed with H 2 O washing. The solvent was then evaporated under reduced pressure and the crude product was purified using silica gel chromatography to give 1-(3,4-dichlorophenyl)-1,5,6,7-tetrahydro-4H-indazol-4-one (5,0 g, 17,79 mmol, 56%, orange solid).
[0257] 1 H RMN (300M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com