Vapor-phase fluorination catalysts for producing HFC-134a and the preparing method
A fluorination catalyst, gas phase fluorination technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc., can solve the limitation of catalyst service life, catalyst deactivation, Crystal form transformation and other problems, to achieve the effect of fast price, high activity, simple operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
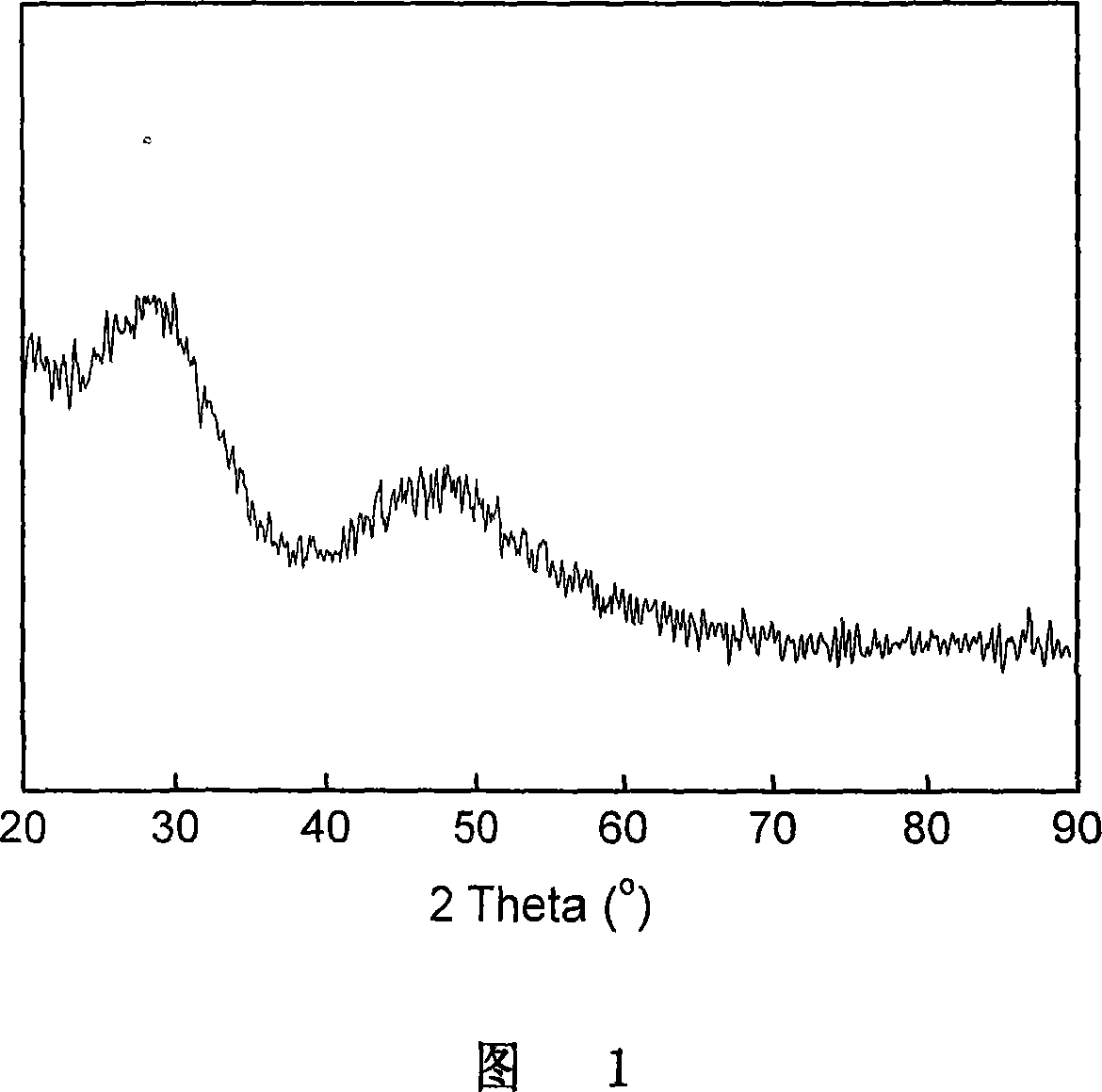
Image
Examples
Embodiment 1
[0019] First, weigh CrCl according to the loading amount of Cr as 30wt%. 3 ·6H 2 O and Y (NO 3 ) 3 ·6H 2 O, dissolve with an appropriate amount of water, mix evenly, add a certain amount of ammonia water, adjust the pH value of the solution to 8, make the two precipitate completely, then centrifuge, wash until the filtrate is neutral, and then dry at 120°C overnight. After that, it is pressed into a tablet and then calcined at 400° C. for 4 hours to obtain a fluorination catalyst precursor. The fluorination catalyst precursor was dried with nitrogen gas at 300° C. for 10 h under normal pressure, and then treated with anhydrous HF at 400° C. for 10 h to obtain a fluorination catalyst. Its reactivity and selectivity are listed in Table 1.
Embodiment 2
[0021] First, weigh Cr(NO 3 ) 3 9H 2 O and YCl 3 ·7H 2 O, dissolve with an appropriate amount of water, mix evenly, add a certain amount of ammonia water, adjust the pH value of the solution to 8, make the two precipitate completely, then centrifuge, wash until the filtrate is neutral, and then dry at 120°C overnight. After that, it is pressed into a tablet and then calcined at 400° C. for 4 hours to obtain a fluorination catalyst precursor. The fluorination catalyst precursor was dried with nitrogen gas at 300° C. for 10 h under normal pressure, and then treated with anhydrous HF at 400° C. for 10 h to obtain a fluorination catalyst. Its reactivity and selectivity are listed in Table 1.
Embodiment 3
[0023] First, weigh Cr(NO 3 ) 3 9H 2 O and Y (NO 3 ) 3 ·6H 2 O, dissolve with an appropriate amount of water, mix evenly, add a certain amount of ammonia water, adjust the pH value of the solution to 8, make the two precipitate completely, then centrifuge, wash until the filtrate is neutral, and then dry at 120°C overnight. After that, it is pressed into a tablet and then calcined at 400° C. for 4 hours to obtain a fluorination catalyst precursor. The fluorination catalyst precursor was dried with nitrogen gas at 300° C. for 10 h under normal pressure, and then treated with anhydrous HF at 400° C. for 10 h to obtain a fluorination catalyst. Its reactivity and selectivity are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com