Type i interferon blocking agents for prevention and treatment of psoriasis
A technology of interferon and blocking agent, applied in the field of type I interferon blocking agent, can solve the problems of not long-term treatment, limited effect of psoriasis, high potential toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
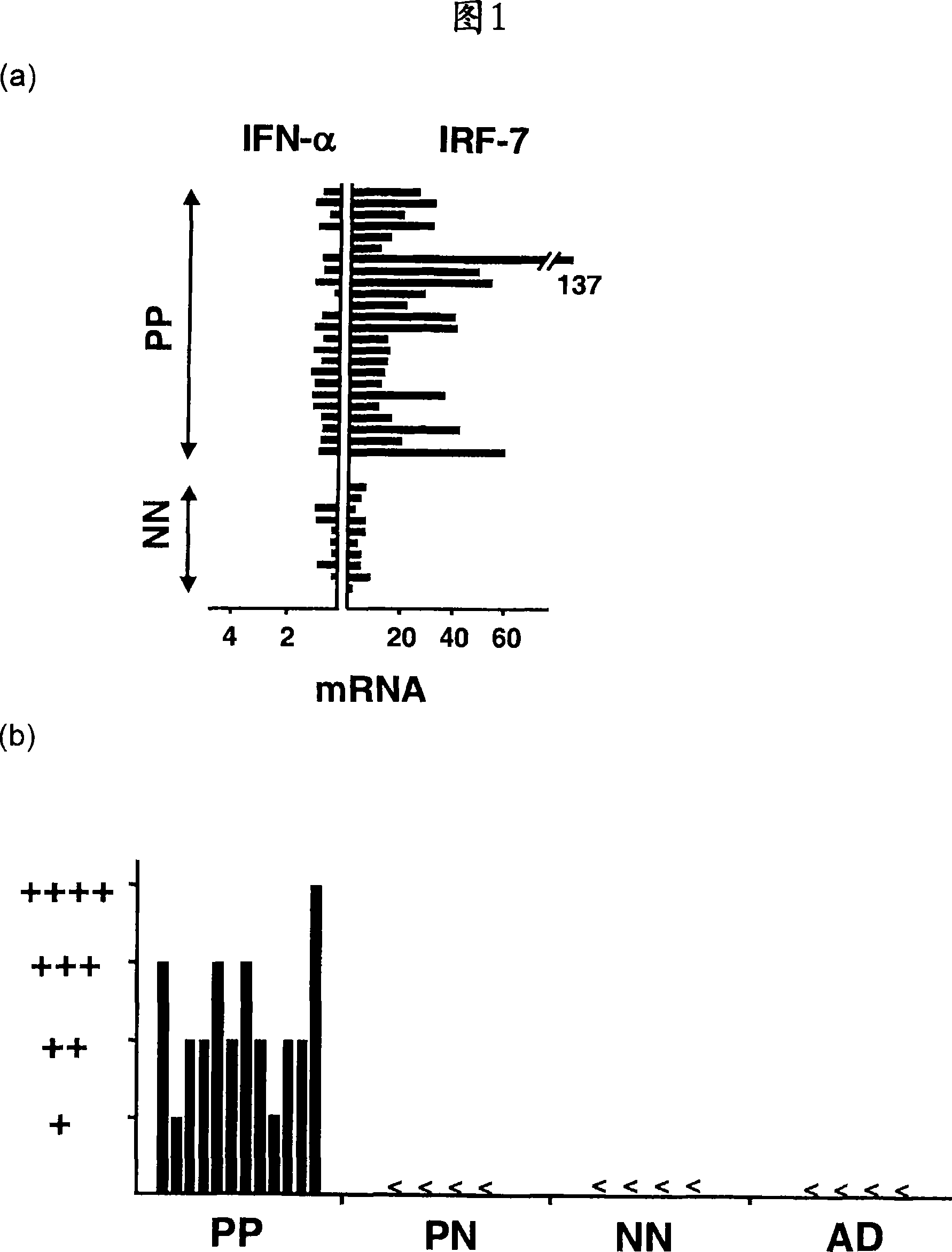
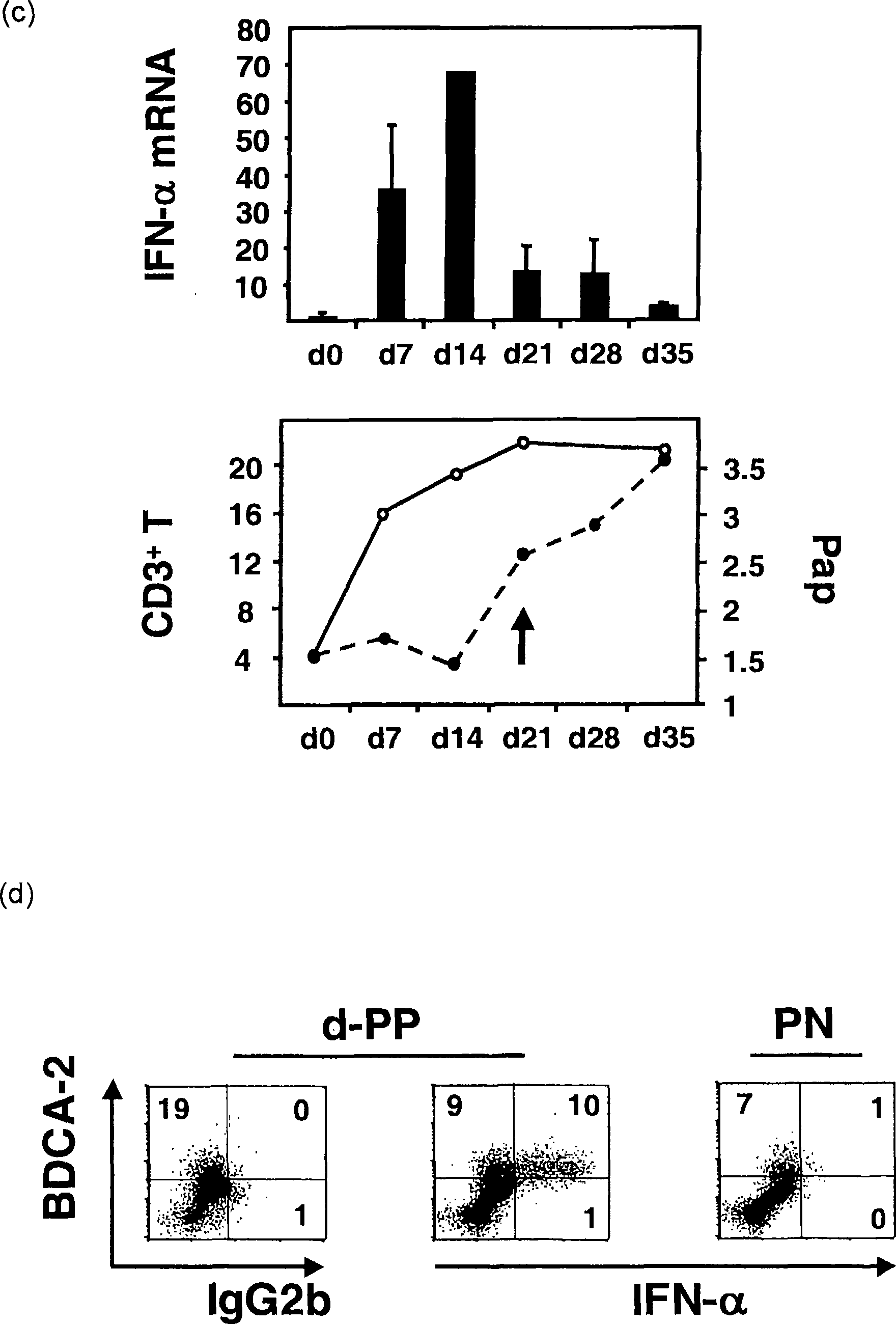
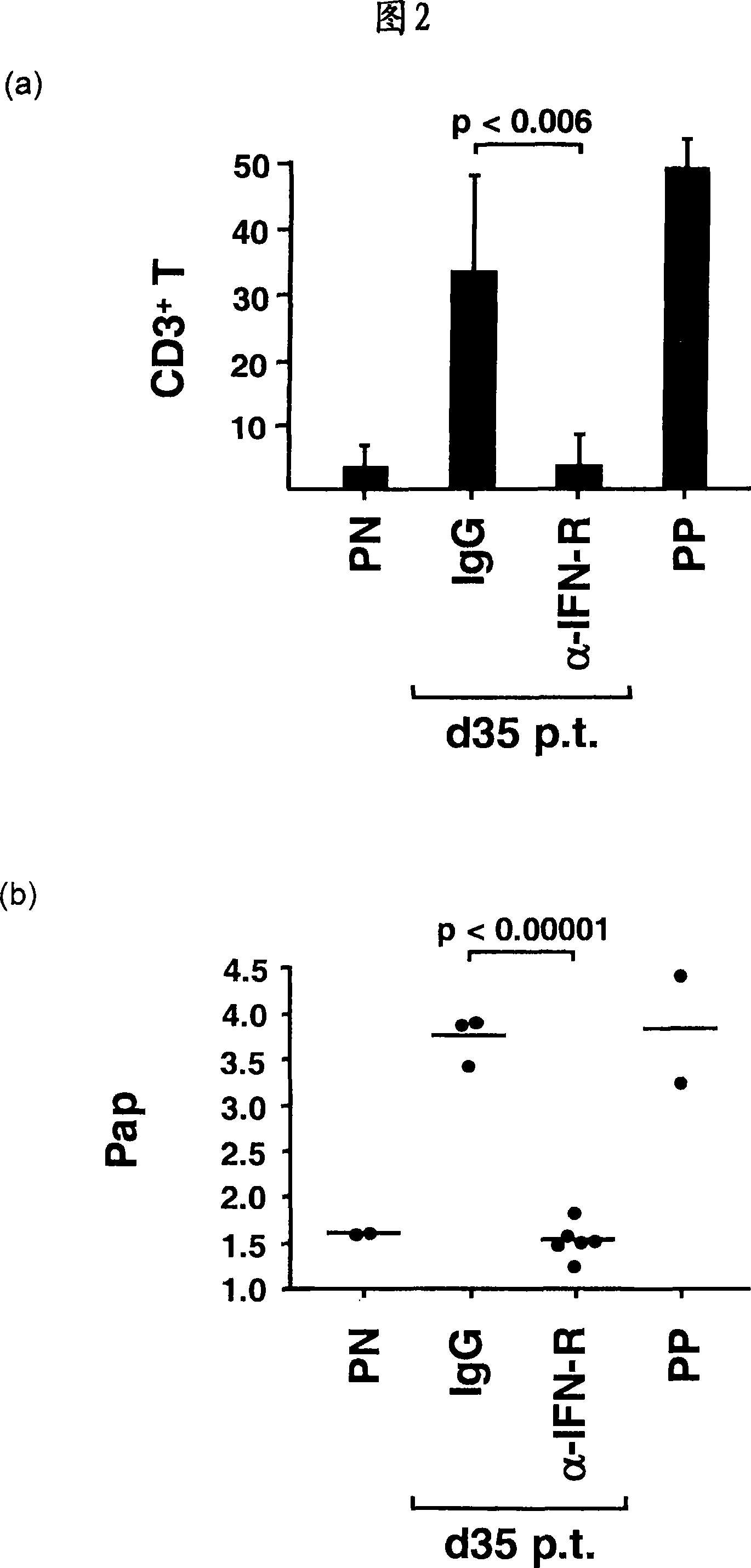
[0041] Quantitative real-time PCR. Total RNA was extracted from homogenized skin samples and reverse transcribed as previously described [Boyman et al., J Exp Med 2004, 199:731-736]. Quantitative analysis by real-time PCR with primers designed against most of the human IFN-α sequences (purchased from Applied Biosystems, Foster City, Canada) and against human IRF-7 (left, TCCCCACGCTATACCATCTACCT-3'; right, ACAGCCAGGGTTCCAGCTT-3') Complementary DNA was used to analyze the expression of IFN-α and IRF-7 transcripts. Normalization was performed with 18S ribosomal RNA. In the humanized mouse model, IFN-α quantification was performed with a primer kit (purchased from Search-LC, Heidelberg, Germany) that recognizes most of the human IFN-α genes but not their mouse counterparts. Human GAPDH mRNA levels were quantified with human-specific primers (left, ATTGCCCTCAACGACCACTTTG-3'; right, TTGATGGTACATGAAAGGTGAGG-3') and used for normalization.
[0042] Animal and Transplantation Procedu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com