CD8 alpha + lymphoid dendritic cell differentiated from human hematopoietic stem cell and a method for differentiation
A technology of dendritic cells and artificial blood, applied in biochemical equipment and methods, blood/immune system cells, animal cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Extraction of hematopoietic stem cells and production of dendritic cells
[0041] In order to activate peripheral blood stem cells (PBSC), granulocyte colony-stimulating factor (G-CSF, Lenograstim, chugai, Co., Tokyo, Japan) was subcutaneously injected into 10 patients with different tumor diseases (breast gland) at a dose of 300 μg / day. cancer, leukemia, lymphoma) patients. Four days after the injection, peripheral blood stem cells were extracted using a Cobe Spectra (Cobe BCT, Inc., Lakewood, CO, USA) cell separator according to the leukocyte extraction method. Using Ficoll-Hypaque (Histopaque, Sigma Chemical, St.Louis, MO, USA), according to the method of density gradient centrifugation, mononuclear cells were separated from the peripheral blood stem cells thus obtained, and washed with phosphate-buffered saline (PBS, Sigma Chemical ) washed twice. The mononuclear cells were filtered through a 30 μm nylon mesh in PBS containing 5% bovine serum albumin (B...
Embodiment 2
[0045] Example 2: Examination of the Immunophenotype of Differentiated DCs
[0046] To confirm the immunophenotype of cultured DCs, 1×10 5 Cells were reacted for 15 minutes with fluorescein isothiocyanate or phycoerythrin-labeled monoclonal antibodies against CD1a, CD3, CD4, CD8α, CD11c, CD14, CD80, CD83, CD86, HLA class I (ABC), HLA class II (DR) (Pharmingen, San Diego, CA, USA), and the resulting solution was washed with PBS, and then analyzed by flow cytometry (FACScan, Becton Dickinson).
[0047] like image 3 As shown in A and 3B, on day 7 of culture, the cells expressed CD1a, CD11c as well as CD40, CD54, CD80, CD86 and HLA class I / II. However, CD83 + , a marker of mature DC, weakly expressed. The addition of ionomycin converted CD1a and CD14 to a negative phenotype, and CD83 + Phenotyped cells increased. In addition, if Figure 4A and 4B As shown, the cells exhibit a negative phenotype for CD3 and CD4, whereas the CD8α phenotype is positive.
[0048] These resul...
Embodiment 3
[0049] Example 3: Confirmation of CD8α + DC's phagocytosis
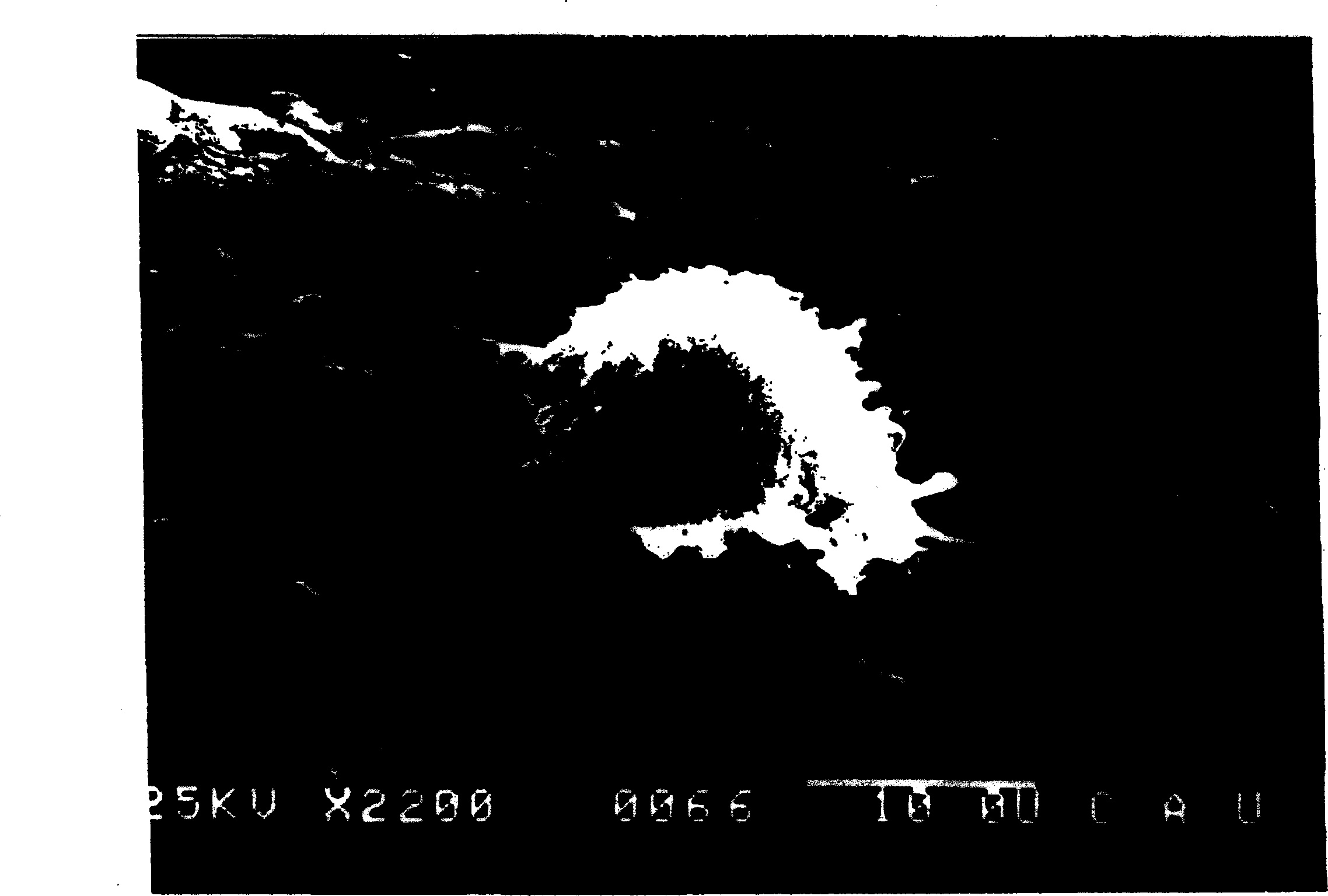
[0050] To confirm the phagocytic ability of differentiated DCs, 2 × 10 5 DC were incubated without FBS with dextran-FITC (Sigma) for 1 hour at 37°C. Cells that had engulfed dextran were then examined by flow cytometry.
[0051] like Figure 5B and 5C As shown, CD8α + CD has a high phagocytic capacity. In addition, on the 14th day of culture, the phagocytic ability was further enhanced. The control experiment (5A) at 4°C shows the observed CD8α of the present invention + The phagocytic activity of CD is real and not an artifact produced by the measurement conditions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com