peg modified cpg oligonucleotide and its application
An oligonucleotide and cpg-7 technology, which is applied in the field of vaccines and tumor immunotherapy delivery systems, can solve the problems of few modified oligonucleotides and low efficiency, so as to increase in vivo stability, improve response, Increased immune modulating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
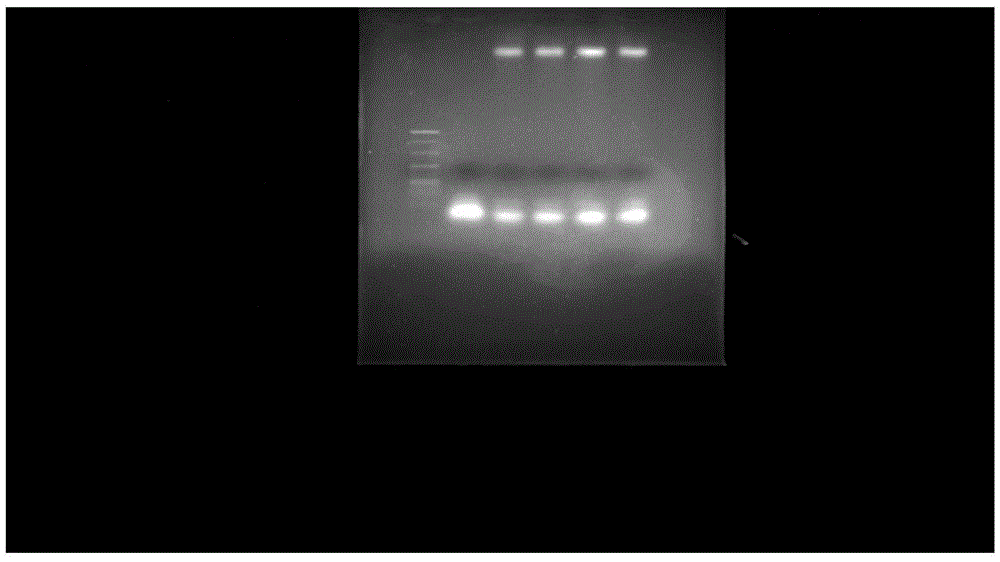
[0065] Embodiment 1, the impact of different reaction times on the synthesis rate of structure 1
[0066] (1) After the ultrapure water is boiled, N 2 After cooling, to remove oxygen, the ultrapure water was used to prepare pH=7.4, 10 mM Tris-HCl as the reaction solvent. Take 20nmol of CpG-SH and dissolve it in 100ul of 10mM Tris-HCl. After dissolving, blow N 2 5 minutes. Weigh 3.5mg of TCEP, add 4ml of 10mM Tris-HCL (pH=7.4) to obtain 3nmol / μl TCEP, blow with nitrogen for 5min, draw 10ul into the EP tube of CPG-SH, and mix well. Weigh 1.0mg of mPEG 20K -mal (other molecular weight increases or decreases in the same proportion) was dissolved in CpG-SH solution, and nitrogen was protected during the whole process. On a constant temperature oscillator at 25°C and 800rpm, react for 5h, 10h, 15h, and 25h, respectively.
[0067] (2) Use agarose gel electrophoresis to compare the difference in synthesis rate of the samples obtained in (1). The results are shown in figure 1 ,...
Embodiment 2
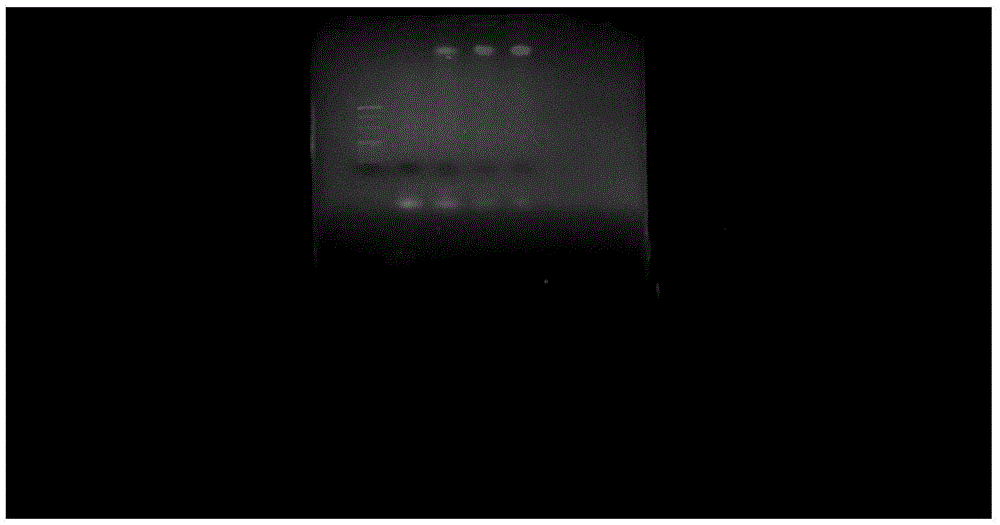
[0068] Embodiment 2, the impact of the feed ratio of CpG-PEG and CpG on the reaction yield of structure 1
[0069] (1) After the ultrapure water is boiled, N 2 After cooling, to remove oxygen, the ultrapure water was used to prepare pH=7.4, 10 mM Tris-HCl as the reaction solvent. Take 15nmol of CpG-SH and dissolve it in 20ul of 10mM Tris-HCl. After dissolving, blow nitrogen gas for 5 minutes. Weigh 3.5mg of TCEP, add 4ml of 10mM Tris-HCl (pH=7.4) to obtain 3nmol / μl TCEP, blow with nitrogen for 5min, draw 10ul into the ep tube of CPG-SH, and mix well. And divided into 3 tubes, weighed and weighed 0.2mg, 0.4mg, 0.6mg of mPEG 20K -mal (other molecular weight increases or decreases in the same proportion) was dissolved in the CpG-SH solution and vortexed. Nitrogen protection during the whole process. React at 25°C and 800rpm on a constant temperature oscillator for 5h respectively.
[0070] (2) Use agarose gel electrophoresis to compare the difference in synthesis rate of t...
Embodiment 3
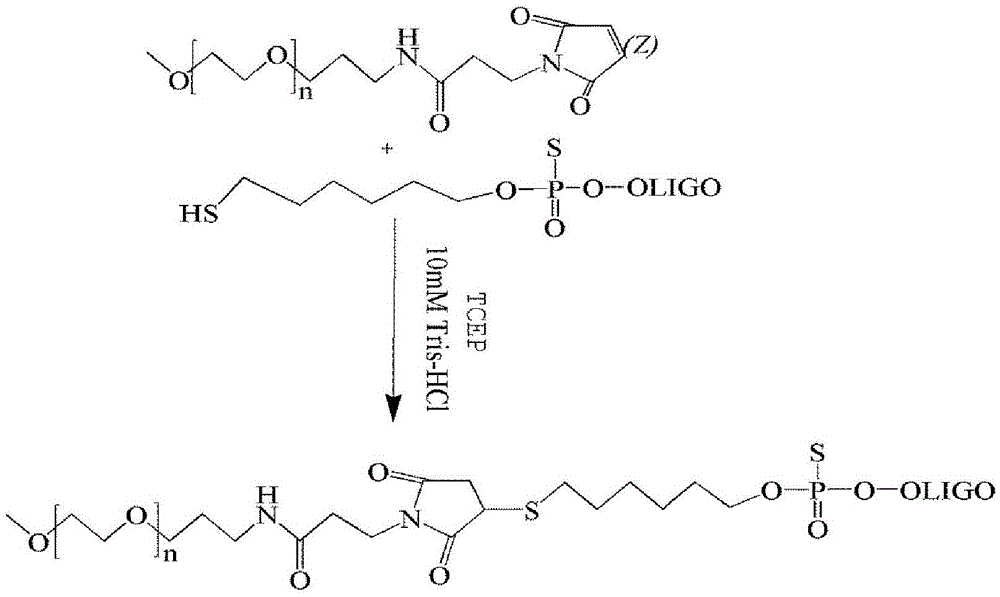
[0071] The synthesis and purification method of CpG-PEG shown in embodiment 3, structure 1
[0072] 1. CpG-PEG 20K synthetic method ( image 3 ).
[0073] (1) After the ultrapure water is boiled, N 2 After cooling, to remove oxygen, the ultrapure water was used to prepare pH=7.4, 10 mM Tris-HCL as a reaction solvent. Take 20 nmol of CpG-SH and dissolve it in 100 ul of 10 mM Tris-HCL. After dissolving, blow nitrogen gas for 5 minutes. Weigh 3.5mg of TCEP, add 4ml of 10mM Tris-HCL (pH=7.4) to obtain 3nmol / μl TCEP, blow with nitrogen for 5min, draw 10ul into the EP tube of CPG-SH, and mix well. Weigh 1.6 mg of mPEG20K-mal (other molecular weights increase or decrease in the same proportion) and add to the above solution, vortex to dissolve completely. N throughout the process 2 Protect. React at 25°C and 800rpm on a constant temperature oscillator for 5h respectively. The dosage can be enlarged or reduced in the same proportion for synthesis.
[0074] (2) Extract the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com