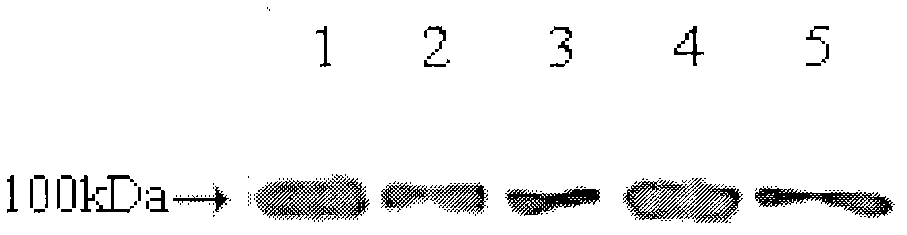Preparation and application of human fibroblast growth factor 21 fusion protein and mutant of human fibroblast growth factor 21 fusion protein
A human fibroblast and fusion protein technology, which is applied in the preparation method of peptides, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., to achieve the effects of improving affinity, reducing injection frequency and reducing pain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
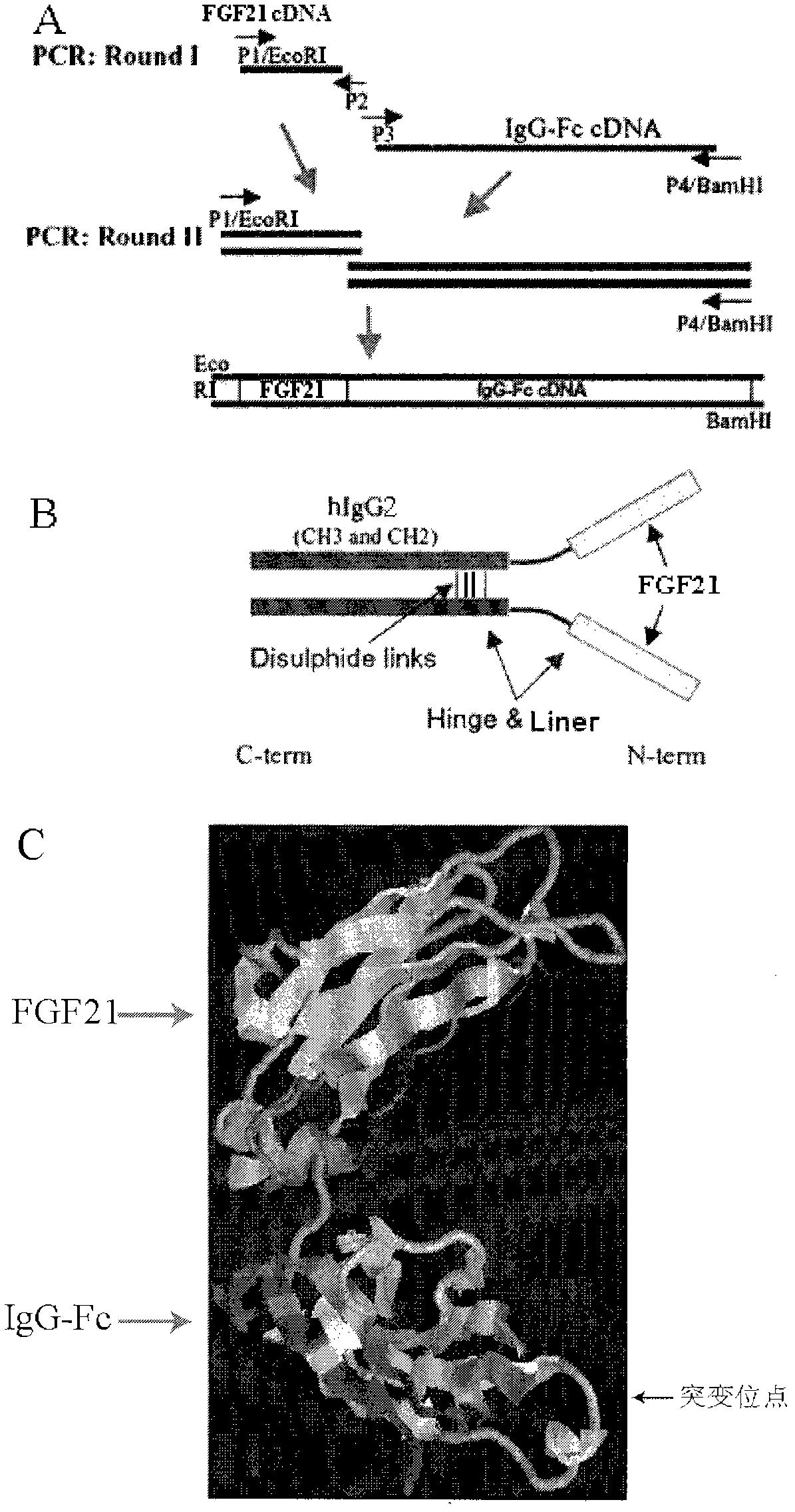
[0068] Embodiment 1: the construction of carrier
[0069] A vector encoding fusion protein containing FGF21 and its mutants and IgG2-Fc and its mutants was constructed by overlapping PCR. The IgG2-Fc region contains an IgG2 constant heavy chain (hinge-CH2-CH3 portion). The murine IgK secretion leader sequence was fused to the FGF21 sequence in order to direct the secretion of the synthetic fusion protein into the culture medium. The cDNA encoding the FGF21 / hIgG-Fc fusion protein was chemically synthesized and ligated to the PCR-amplified product encoding human IgG2-Fc (hinge, CH2, and CH3), then inserted into the EcoRV and BamHI of the pcDNA3.1 vector The FGF21 / hIgG-Fc-pcDNA3.1 vector was constructed between the sites. Secretable FGF21 / hIgG-Fc and its mutant fusion proteins contain an IgG2 constant heavy chain (hinge-CH2-CH3). The ERBITUX heavy chain secretion guide peptide sequence (IGH) is fused to the FGF21 sequence to guide the secretion of the synthesized protein into ...
Embodiment 2
[0076] Example 2: CHO expression of FGF21 / IgG-Fc and its mutant fusion proteins
[0077] FGF21 / IgG-Fc or IgG-Fc cDNA was transfected into CHO-S cells by Lipofectamine 2000 (Invitrogen, Carlsbad, CA). The process is to first make the density 2.5×10 per well 5 Individual CHO-S cells were grown in 6-well cell culture plates. Serum-free and antibiotic-free DMEM (Invitrogen) containing 4 µg of FGF21 / IgG-Fc cDNA and 10 µl of transfected liposomes was added for culture. After 6 hours of transfection, switch to full culture medium. Culture medium and cells were collected 48h after transfection. In order to express FGF21 / IgG-Fc fusion protein on a large scale, CHO-S cells grown in a 150mm culture dish were related with cationic transfection reagent, poly-ethyleneimine (Poly-ethyleneimine; PEI, 25kDa) and 80μg. cDNA transfection. Dilute DNA and PEI with 150mM Nacl respectively, mix and incubate for 20min. Then the DNA / PEI complex was added to the cells and incubated for 6 h in ser...
Embodiment 3
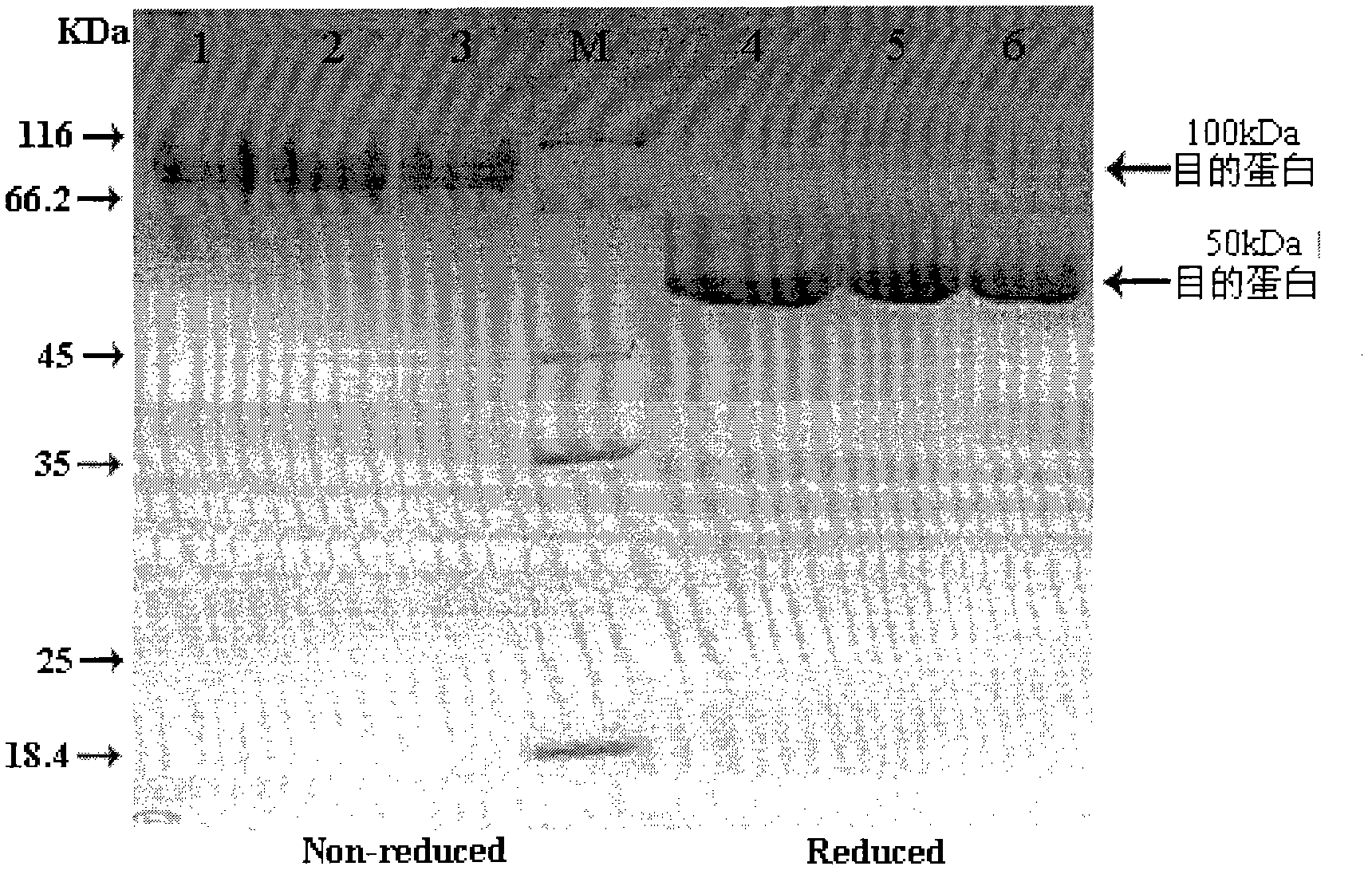
[0079] Example 3: Purification of fusion proteins from mammalian cell cultures
[0080] Medium-scale protein purification can be performed using a Sephadex A column. A column volume of 50 ml can purify the conditioned medium of CHO-S cells transfected with FGF21 / IgG-Fc and its mutant fusion vector grown in 15 cm dishes. 50 ml of DMEM culture medium at 48 h after cell transfection, or cells stably expressing fusion protein were collected and added to a 1 mL full volume of Protein A Sephadex (Amersham-Pharmacia). Add 1% TritonX-100 and incubate overnight at 4°C. The protein was then eluted from the resin by washing with 1% TritonX-100 in PBS buffer, then with 150 mM NaCl, and finally with 1 mM NaCl glycine (pH 2.7). The eluate was immediately neutralized with Tris buffer (pH 9.0) and the purified protein was desalted with a PD-10 gel column (Amersham-Pharmacia) and eluted with PBS. As seen in the figure ( figure 2 ), the two-step elution method can elute most of the fusion pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com