COP1 molecules and uses thereof
A molecular, versatile technology with applications in the field of cancer diagnosis and therapy that can address poorly defined problems as
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1: Materials and methods
[0110] Expression Vectors, Recombinant Proteins and Antibodies
[0111] Flag-COP1 has been described earlier 33 . HA-COP1 was generated by PCR subcloning COP1 into pcDNA3.1+ (Invitrogen) and GST-COP1 was generated by subcloning COP1 into pGEX6P1 (Pharmacia). pcDNA3.1+53, pG13-Luc, p21-Luc, bax-Luc, NS-Luc and pCMV-MDM2 have been described earlier 21,22,35 .
[0112] Full-length COP1 was amplified from cDNA from HEK293T cells. HA-COP1 was subcloned by PCR into pcDNA3.1+ (Invitrogen) and GST-COP1 was generated by PCR and subcloned into pGEX6P1 (Pharmacia). COP1-Luc and COP1mut-Luc were generated by ligating oligonucleotides containing two copies of the p53 consensus site from the COP1 promoter, or a mutant containing the consensus site, into the pGL3-promoter (Promega).
[0113] All GST recombinant proteins were expressed in E. coli BL21(DE3) codon+ (Stratagene), sonicated with 1 mg / ml lysozyme, dissolved in PBS with 1% TritonX-100...
Embodiment 2
[0164] Example 2: p53 is a substrate of COP1
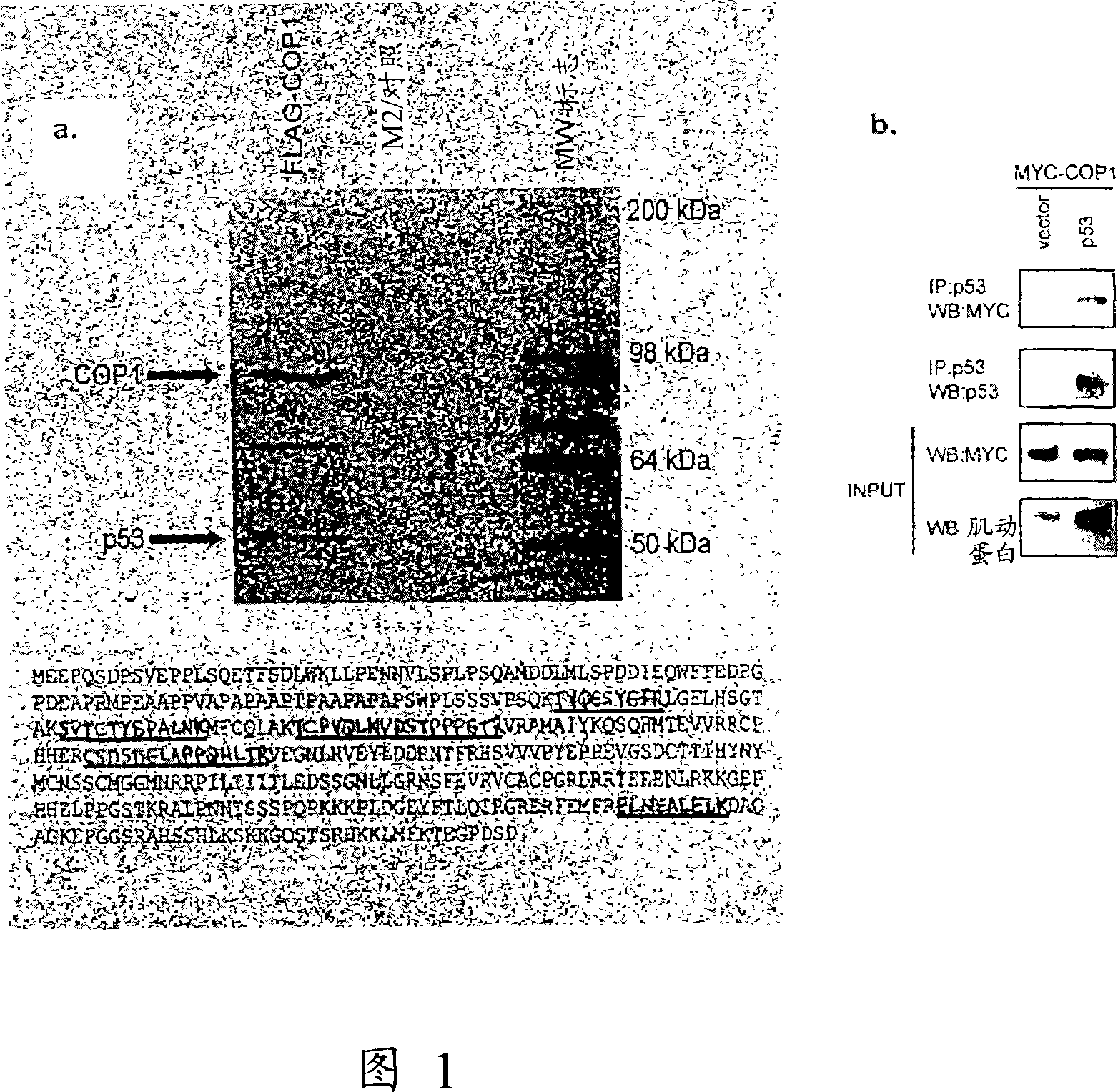
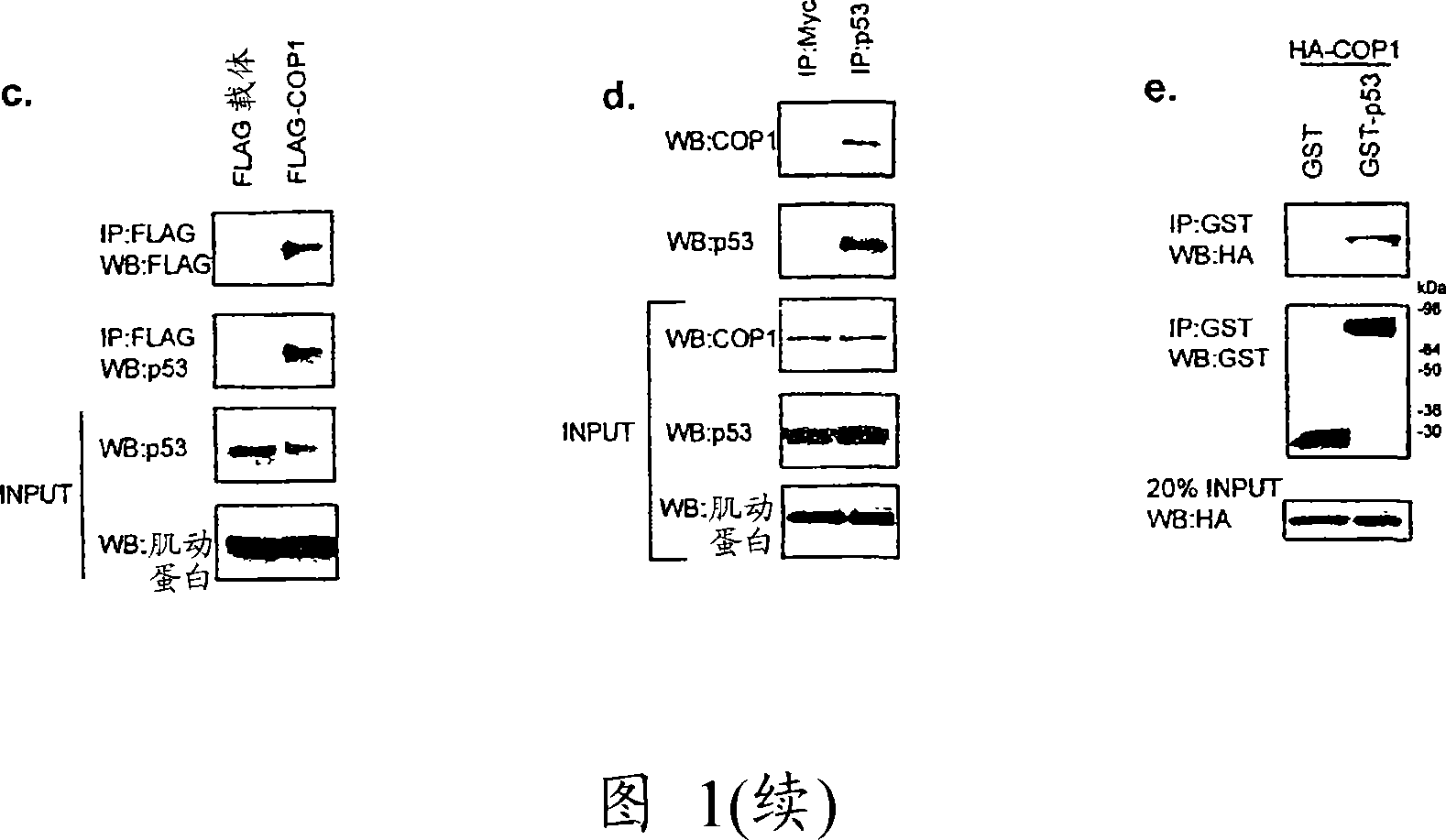
[0165] To gain insight into how COP1 regulates cellular processes, lysates from U2-OS cells stably expressing FLAG-tagged COP1 or empty vector were subjected to immunoprecipitation with anti-FLAG, bound proteins were eluted by FLAG peptide, and bound proteins were eluted by SDS - PAGE analysis, sequencing specific bands by mass spectrometry (Fig. 1A). Mass spectrometric analysis of the band at approximately 53 kDa revealed 5 matching peptides from the tumor suppressor protein p53. To confirm that this interaction was real, p53-null Soas-2 cells were transfected with p53 and Myc-COP1, immunoprecipitated with anti-p53 (DO-1), and immunoblotted with anti-Myc (Fig. 1B). COP1 was immunoprecipitated only in the presence of transfected p53, thus suggesting that COP1 can interact with p53. This interaction was also observed with transfected COP1 and endogenous p53 (Fig. 1C). Furthermore, there was an interaction between p53 and COP1 at...
Embodiment 3
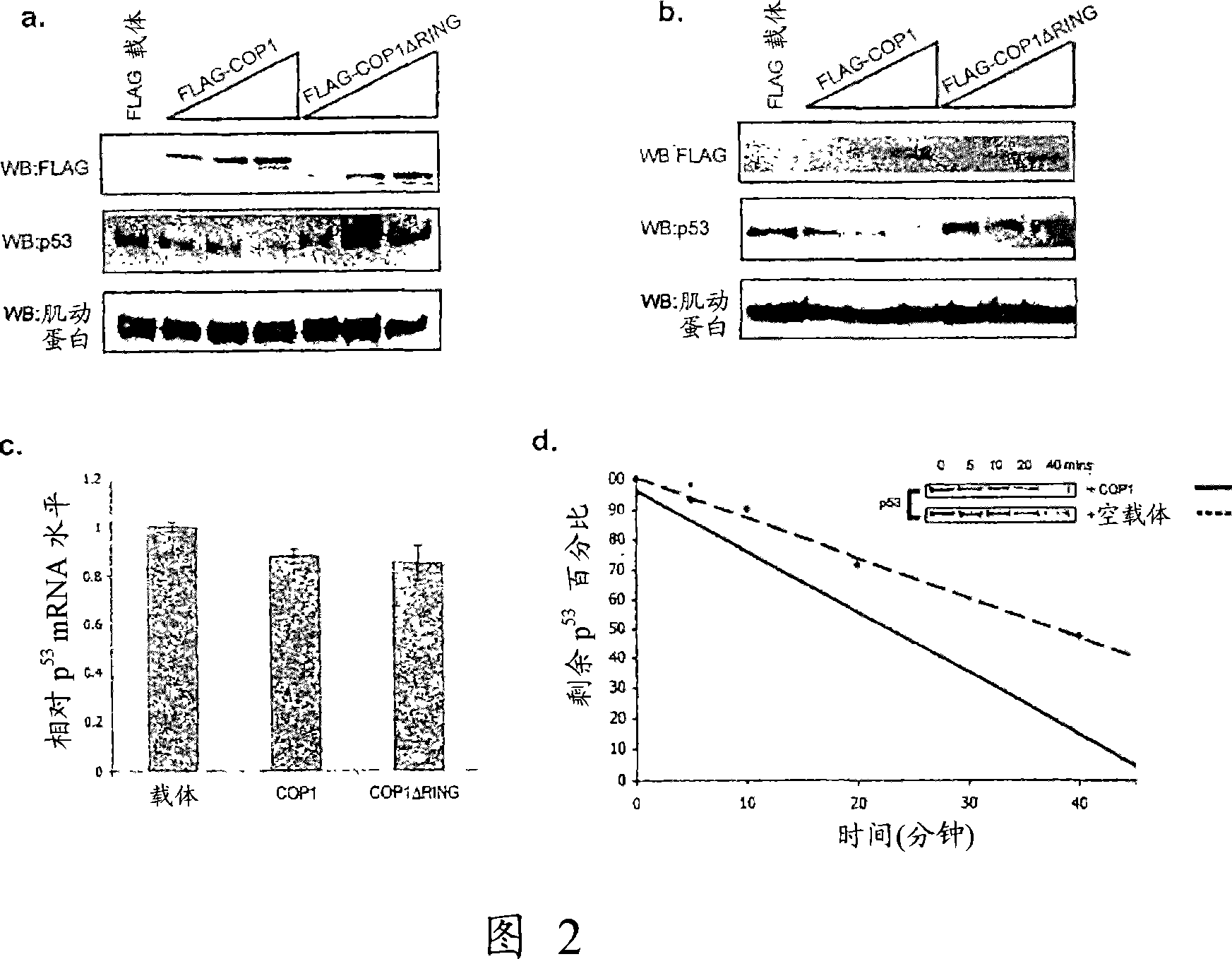
[0173] To determine the effect of COP1 overexpression on p53-dependent transactivation, Saos-2 cells were transfected with p53 and COP1 or COP1ΔRING, and p21-luciferase (Luc) or bax-Luc (Fig. 2H, L). Addition of COP1 markedly reduces the ability of p53 to transactivate from the p21 and bax promoters. Furthermore, the transactivation capacity of endogenous p53, as well as the capacity induced by DNA damage, was abrogated in the same manner by overexpression of COP1 (Fig. 2M). To further assess the ability of COP1 to negatively regulate p53, we determined whether COP1 could inhibit p53-induced cell death in Saos-2 cells (Fig. 21). Transfection of p53 significantly increased the cell population in the SubG1 population, however, this was significantly inhibited by co-transfection of COP1, suggesting that COP1 inhibits p53-induced cell death. COP1 transfection alone had no profound effect on cell cycle distribution.
[0174] To reveal the role of COP1 in more physiological situat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com