Mini protein and its application
A mini protein and mini technology, applied in the field of mini protein, can solve the problems of metabolic excretion, short circulation time of inhibitors, inability to exert drug effect, etc., and achieve the effect of inhibiting interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
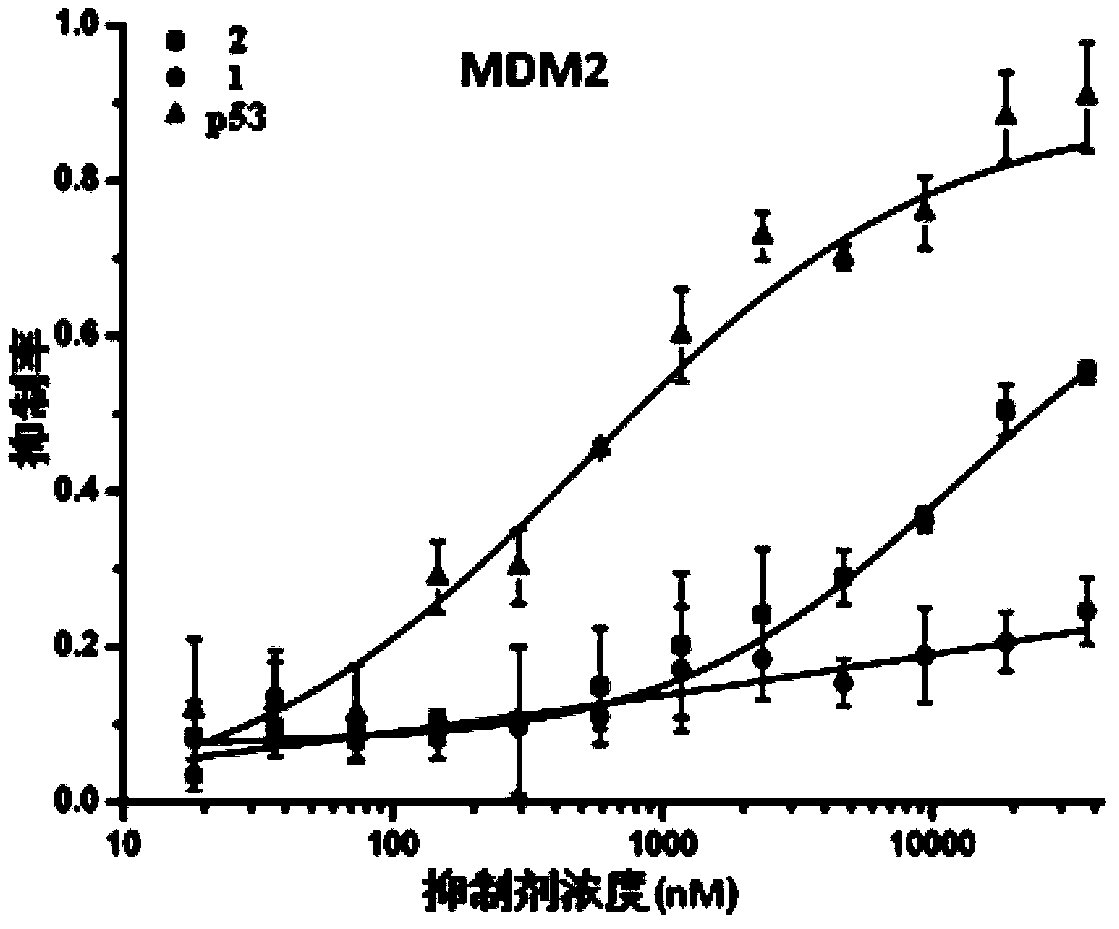
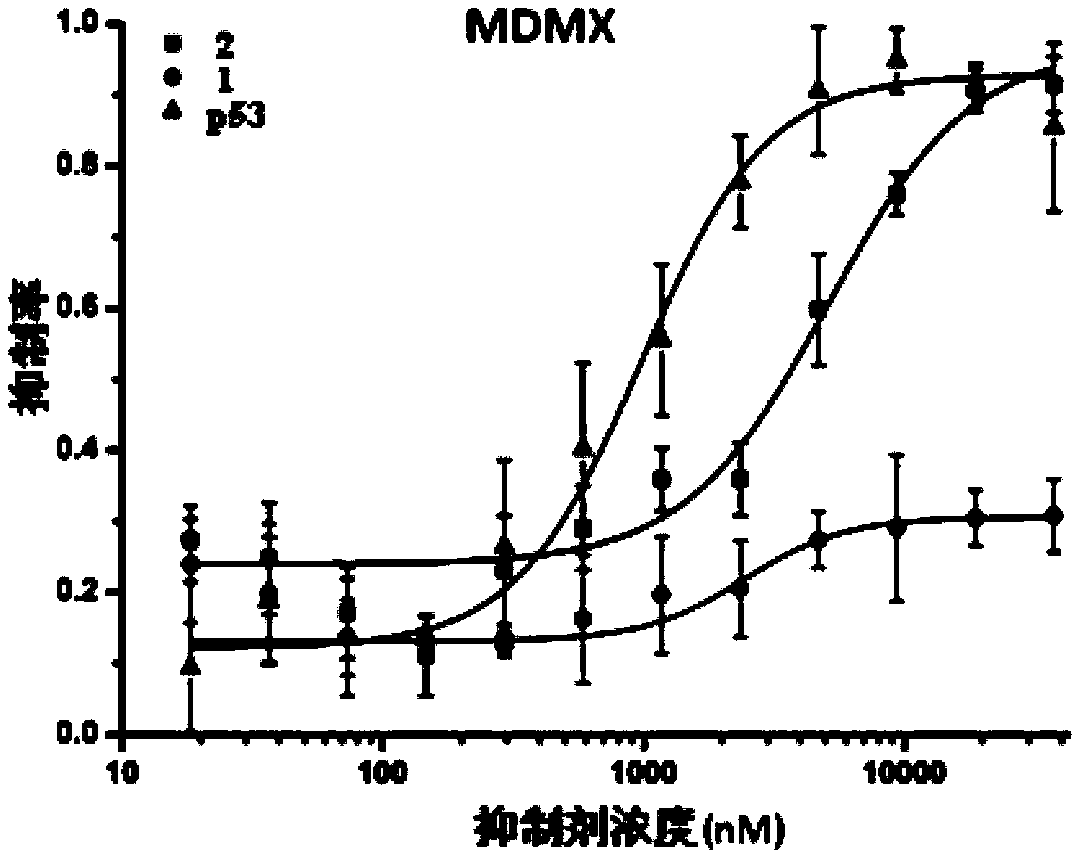
[0035] Example 1 Detection of binding of wild-type and small proteins grafted with key amino acids to MDM2 / MDMX
[0036] Take the mixture containing 50nM FITC-p53 and 1uM MDM2 / MDMX, dilute the wild-type (No. 1) and key amino acid-grafted small protein (No. 2) and p53 polypeptide from 37.5uM to 12 concentrations respectively, and mix with the aforementioned mixture After interacting with each other for half an hour, the fluorescence polarization intensity was detected on a microplate reader, and the inhibitory concentration between the aforementioned small protein and MDM2 / MDMX was calculated. The results can be seen in Figure 2.
Embodiment 2
[0037] Example 2 Affinity detection between wild-type and small proteins grafted with key amino acids and albumin
[0038] First, immobilize albumin HSA (20ug / ml) on the CM5 chip, and prepare the No. 1 and No. 2 small proteins described in Example 1 at concentrations of 0, 0.2ug / ml, 0.4ug / ml, and 0.8ug / ml, 1.6ug / ml, and 3.2ug / ml solutions, the buffers used in these solutions are all HBS-EP.
Embodiment 3
[0039]Example 3 Helical Structure Determination of Various Parvalbumin Introduced by Wild Type, Key Amino Acid Grafting, Key Amino Acid Grafting, and Cationic Amino Acids
[0040] Prepare No. 1, No. 2 protein, No. 3, No. 4, and No. 5 small protein introduced by key amino acid grafting and cationic amino acid into a solution with a concentration of 0.4 mg / ml in PBS buffer, and place in circular dichroism On the spectrometer, detect its α helix, and the test results can be found in Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com