Multifunctional anticancer recombinant adenovirus
A recombinant adenovirus and virus technology, applied in virus/bacteriophage, recombinant DNA technology, anti-tumor drugs, etc., can solve the problems of increased pain for patients, poor tumor efficacy, increased toxicity of chemotherapy drugs, etc., to reduce toxicity response, increasing the effect of the maximum tolerated dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
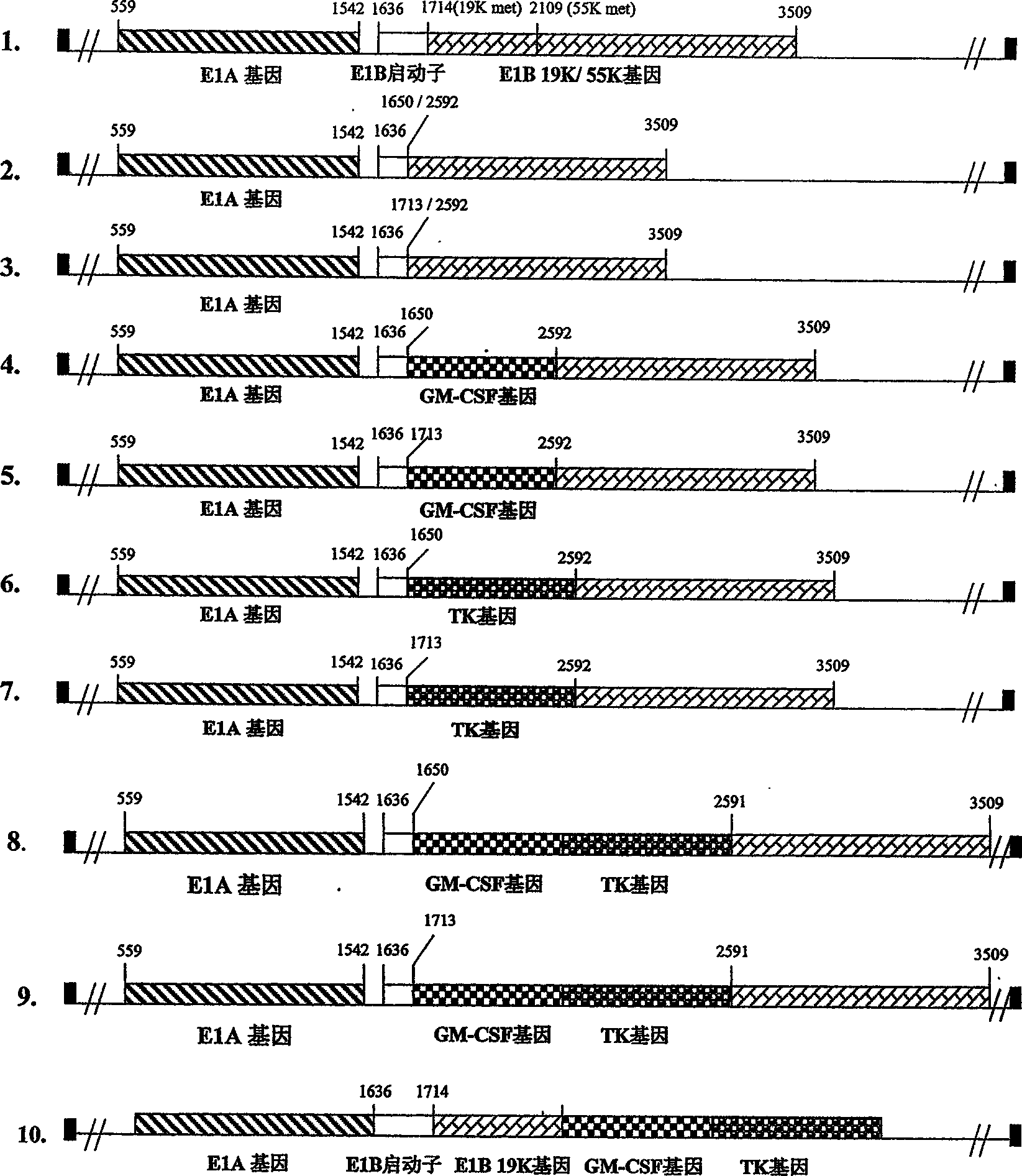
[0071] Example 1. Construction of Adenovirus E1B Gene Mutants
[0072] A. Construction of plasmid pXC-Δ1651
[0073] Plasmid pXC-1 was purchased from Microbix Biosystem Inc (Toronto), Canada. pXC-1 contains the gene sequence of human adenovirus type 5 (Ad5) from nt22-5790. An adenovirus left-end plasmid with deletion of E1B gene (from nt1651-2591) was constructed on pXC-1 by PCR method [refer to Molecular Cloning for detailed experimental steps: Experimental Manual, Second Edition, edited by Sambrook (1989)]. The specific construction steps are as follows:
[0074] oligonucleotide chain
[0075] UCP 1 (5'-TGAGACGCCCGACATCACCT-3')
[0076] UCP2 (5'-CCG CTCGAG CGG TTAATTAA CCTAACACGCCATGCAAGTTAA-3')
[0077] XhoI PacI was used as a primer, and pXC-1 DNA was used as a template to obtain PCR product A. PCR product A contains the gene sequence of adenovirus from nt1316-1650 and contains oligonucleotides at the 3' end
[0078] CC AATTAATT GGC GAG...
Embodiment 2
[0117] Example 2. Construction of transgenic E1B gene mutants
[0118] A. Construction of plasmid pXC-Δ1651 / GM-CSF
[0119] Take the oligonucleotide chain:
[0120] UCP5 (5'-CGC GGATCC GCGATGTGGCTGCAGAGCCTGCT-3')
[0121] BamHI
[0122] UCP6 (5'-CC GGAATT CCCCTCACTCCGGACTGGCTCCC-3')
[0123] EcoRI was used as a primer, and the plasmid PGT60hGM-CSF (purchased from Invivogen, USA) was used as a template to obtain PCR product F. PCR product F contains the complete human-derived GM-CSF gene sequence, and contains restriction sites of BamHI and EcoRI at the 5' end and 3' end. The PCR product F was digested with EcoRI and BamHI, and inserted into the plasmid pcDNA3 (purchased from Invitrogen, USA) at the same site to obtain a new recombinant plasmid pcDNA3-GM-CSF.
[0124] Take the oligonucleotide chain:
[0125] UCP7 (5'-CC TTAATTAA GGGTTGACATTGATTATTGACT-3')
[0126] PacI
[0127] UCP8 (5'-CC TTAATTAA GGATGCAATTTCCTCATTTTATT-3') ...
Embodiment 3
[0159] Example 3. Production of recombinant adenovirus
[0160] A. Generation of Δ1651 adenovirus
[0161] pBHGE3 was purchased from Microbix Biosystems Inc, Canada. pBHGE3 contains the Ad5 gene sequence but the E1 region is deleted from nt188 to 1339. pBHGE3 itself is not infectious, but pXC-1 or a plasmid derived from PXC-1 is co-transfected with pBHG-E3 into 293 cells, and an infectious virus can be produced through homologous recombination (see Hitt for detailed experimental steps, Construction and Propagation of Human Adenovirus Vectors, In: Cell Biology: ALaboratory Handbook, J. Celis, Academic Press, NY, 1995, or refer to Graham, Adenovirus Based Expression Vectors and Recombinant Vaccines, In: Vaccines: New Approaches to Immunological Problems. R.W. Eliss, Butterworth, pp363 -390, 1992). Δ1651 adenovirus was obtained by co-transfecting 293 cells with plasmid pXC-Δ1651 and plasmid pBHGE3 by homologous recombination. Single plaques were picked and amplified in 293 ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com