Use of flagellin in tumor immunotherapy
A flagellin, tumor technology, applied in the field of use of flagellin in tumor immunotherapy, can solve problems such as the limitation of effective biological activity range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Cancer antigens and their vaccines
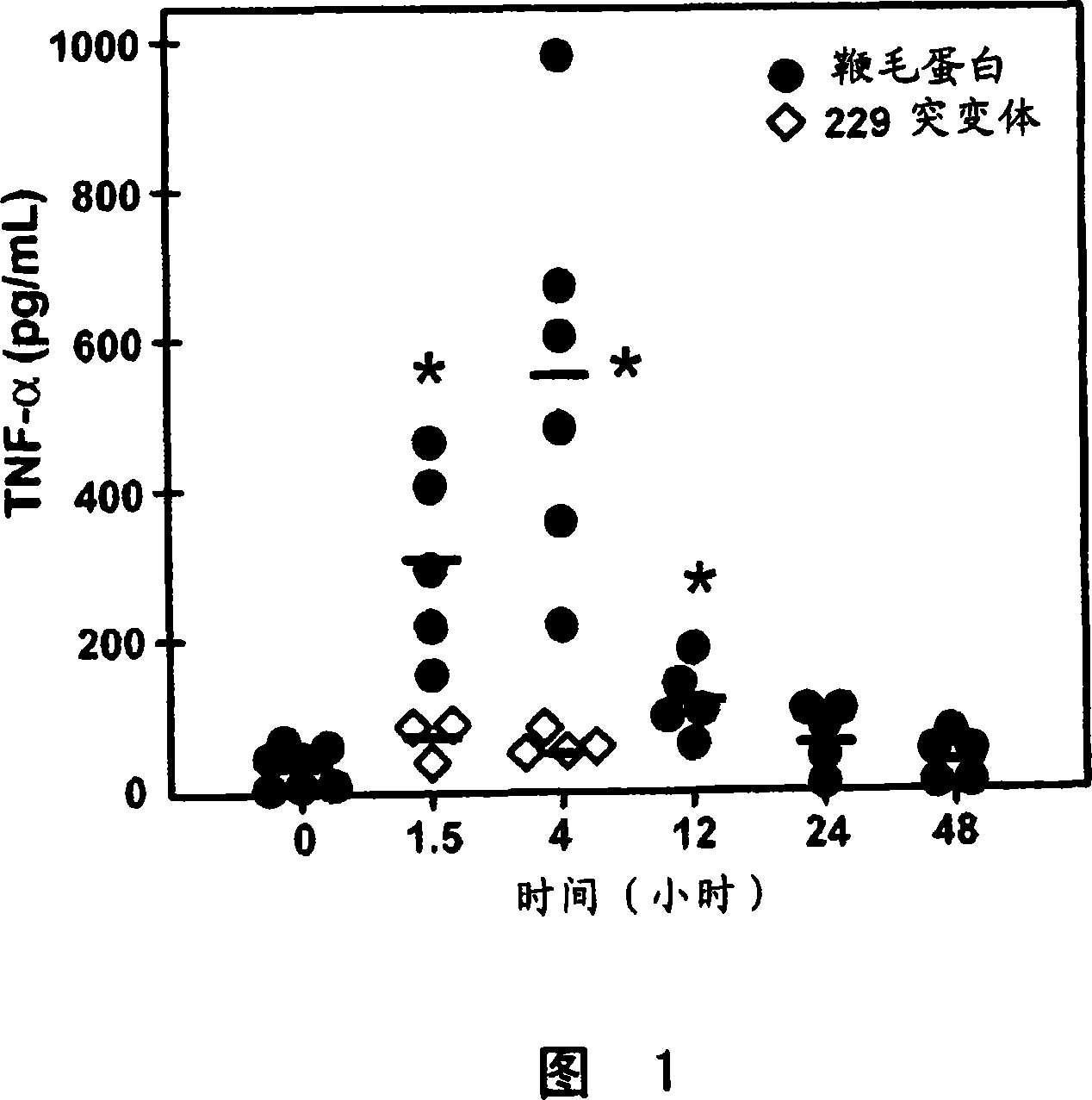
[0136] Effects of flagellin on innate immunity in the mouse lung. A non-surgical intratracheal (i.t.) instillation of 1 μg flagellin was sufficient to induce maximal production of TNF[alpha] after about 4 hours (Figure 1). Cytokine levels in bronchopulmonary lavages returned to baseline levels after 12-24 hours. NOTE: Mutated flagellin that does not bind TLR5 and thus lacks signaling activity does not induce cytokine production. In addition to TNF[alpha], several other cytokines (including IL-6, G-CSF) and chemokines MIP-2 and KC were induced to relatively high levels. An increase in cytokine expression was followed by a transient infiltration of neutrophils (maximum at 12-24 hours). It is important to emphasize that the innate immune response elicited by flagellin does not lead to severe tissue-damaging inflammation. The induced inflammatory response is relatively modest and acute in nature. Together, these findings and those...
Embodiment 2
[0141] Flagellin as an adjuvant to generate antigen-specific breast cancer responses
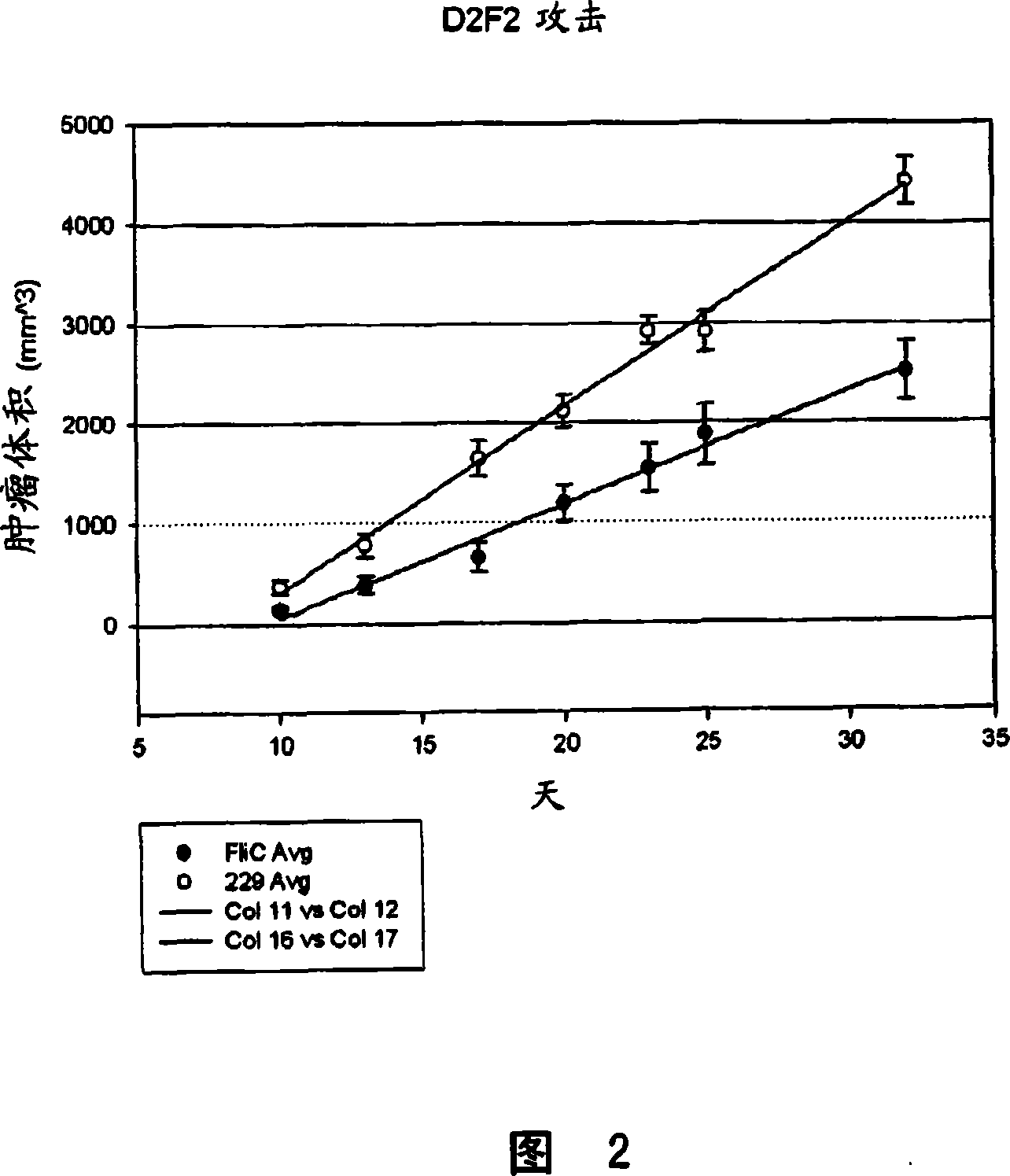
[0142] In vivo treatment of breast tumors. BALB / c mice were immunized with Fra-1 (an antigen overexpressed in many mammary tumors), flagellin, or an inactive form of flagellin. Mice were boosted once before subcutaneous injection of a breast cancer tumor D2F2 cells. Mice were monitored for tumor growth and tumor volumes were determined. The data are shown in Figure 2. Open circles represent mice administered Fra-1 antigen and an inactive form of flagellin. Solid circles represent mice administered Fra-1 antigen and active form of flagellin. It is evident from the figure that the flagellin+Fra-1 antigen has a significant effect on tumor growth.
[0143] To assess whether flagellin can promote a protective immune response against D2F2 breast cancer cells in a mouse model. The ability of flagellin to promote the protective status of immunity against D2F2 breast cancer cells was evaluate...
Embodiment 3
[0155] Flagellin targets Yersinia pestis
[0156] Potent mucosal adjuvant for immunity to lethal respiratory challenge
[0157] method:
[0158] Plasmids and cell cultures. The coding sequence caf1 (plasmid containing the entire caf operon, kindly provided by Dr. J.B. Bliska (State University of New York, Stony Brook)) of the F1 antigen coding sequence of Yersinia pestis was subcloned into a protein from Novagen (EMDBiosciences, Inc. , Madison, WI) in the NdeI and XhoI sites of the pET29a expression vector. The recombinant F1 / V fusion construct (Heat et al. (1998) Vaccine 16: 1131-1137) (provided by Drs. G. Andrews and P. Worsham, USAMRIID) was sequenced and subcloned into pET16b. Sequencing revealed that the 21 amino acids corresponding to the signal sequence of F1 were absent.
[0159]Reagents and Antibodies. Purified, Recombinant, His-tagged flagellin. 229 mutated flagellin as well as F1 and F1 / V antigens were purified in the same manner. Endotoxin levels were <...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com