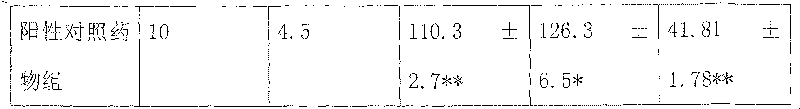Medicine composition for treating chronic active hepatitis and early-stage hepatocirrhosis and preparation method thereof
A technology of active hepatitis and composition, which is applied in the field of traditional Chinese medicine, can solve the problems of low HBsAg negative conversion rate, long taking time, large side effects, etc., and achieve the effect of scientific and reasonable composition, easy acceptance and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
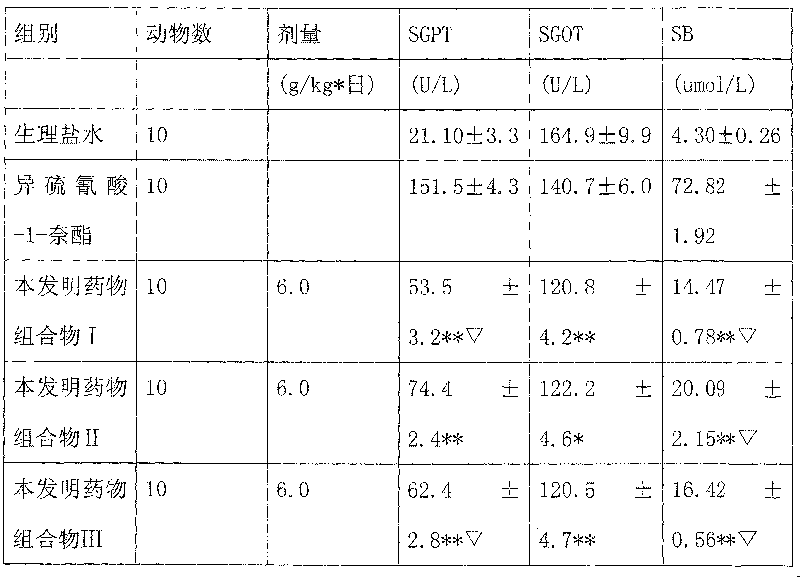
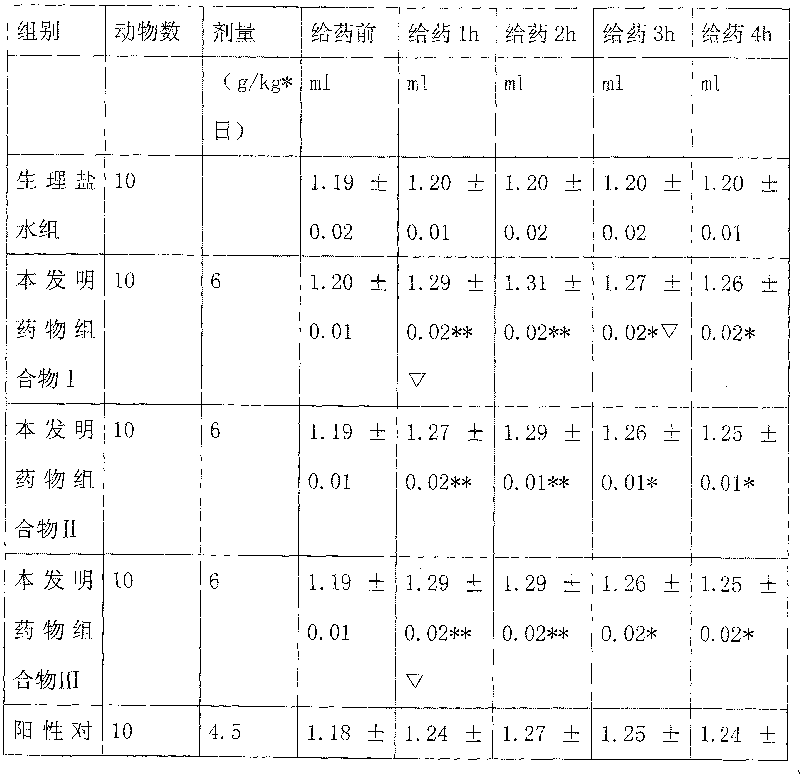
Examples
Embodiment 1
[0115] Yinchen 400g Banzhilian 400g Poria cocos 400g Curcuma 100g Zeilan 200g
[0116] Safflower 100g Bupleurum 100g Amber 40g White Peony 400g
[0117] The above nine flavors are decocted with water for 1-3 times, each time for 1-3 hours, the decoctions are combined, concentrated to a clear paste with a relative density of 1.20-1.35 (50°C), and ethanol is added to make the alcohol content reach 60-70%. Stand still for 20-48 hours, recover ethanol, concentrate to a relative density of 1.25-1.40 (50°C), add appropriate amount of auxiliary materials, and prepare various preparations for clinical application, such as: granules, capsules, tablets, oral liquids, soft drinks, etc. Capsules, dropping pills, dispersants, effervescent agents, sustained-release preparations, etc.
Embodiment 2
[0119] Yinchen 400g Banzhilian 400g Poria cocos 400g Curcuma 100g Zeilan 200g
[0120] Safflower 100g Bupleurum 100g Amber 40g White Peony 400g
[0121] The above nine flavors are decocted twice with water, each time for 2 hours, the decoctions are combined, concentrated to a clear paste with a relative density of 1.30 (50°C), and ethanol is added to make the alcohol content reach 65%, and the ethanol is recovered after standing for 24 hours. Concentrate to a relative density of 1.35 (50°C), take 1 part of clear paste, 3 parts of sucrose, and 1 part of dextrin, make granules, dry, and obtain;
Embodiment 3
[0123] Yinchen 100g Banzhilian 100g Poria cocos 100g Curcuma 25g Zeilan 50g
[0124] Safflower 25g Bupleurum 25g Amber 10g White Peony 100g
[0125] The above nine flavors are decocted twice with water, each time for 2 hours, the decoctions are combined, concentrated to a clear paste with a relative density of 1.30 (50°C), and ethanol is added to make the alcohol content reach 65%, and the ethanol is recovered after standing for 24 hours. Concentrate to a relative density of 1.35 (50°C), take 1 part of clear paste, 3 parts of sucrose, and 1 part of dextrin, make granules, dry, and obtain;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com