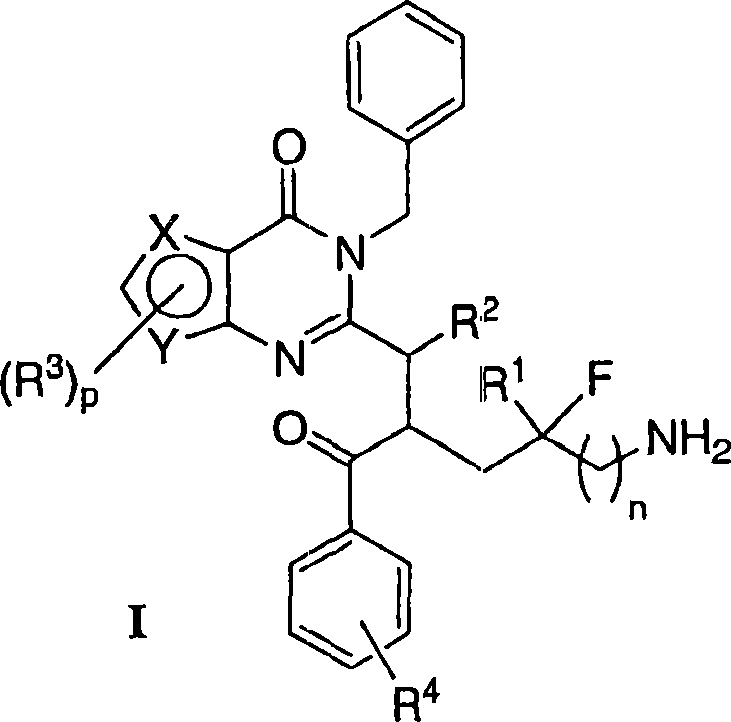
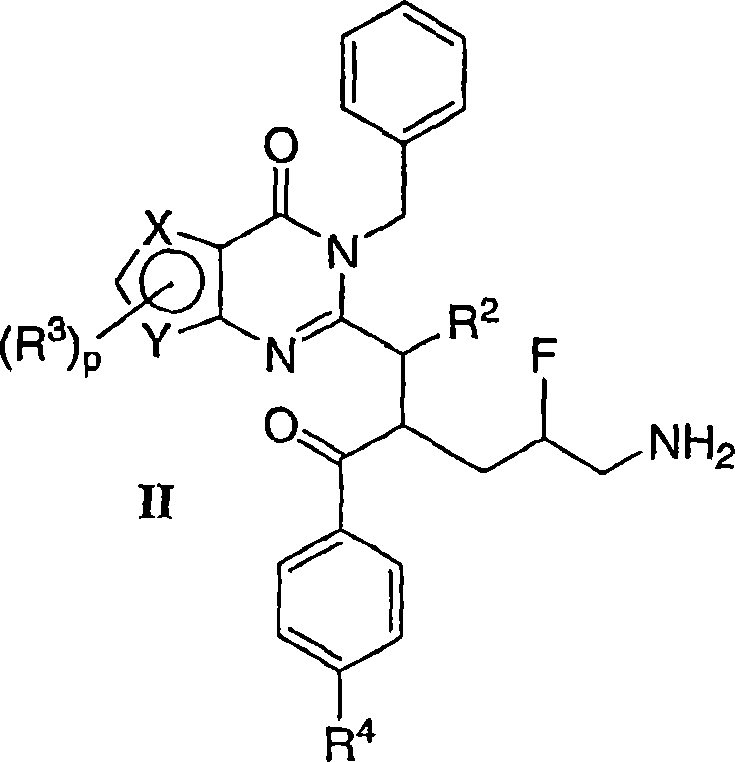
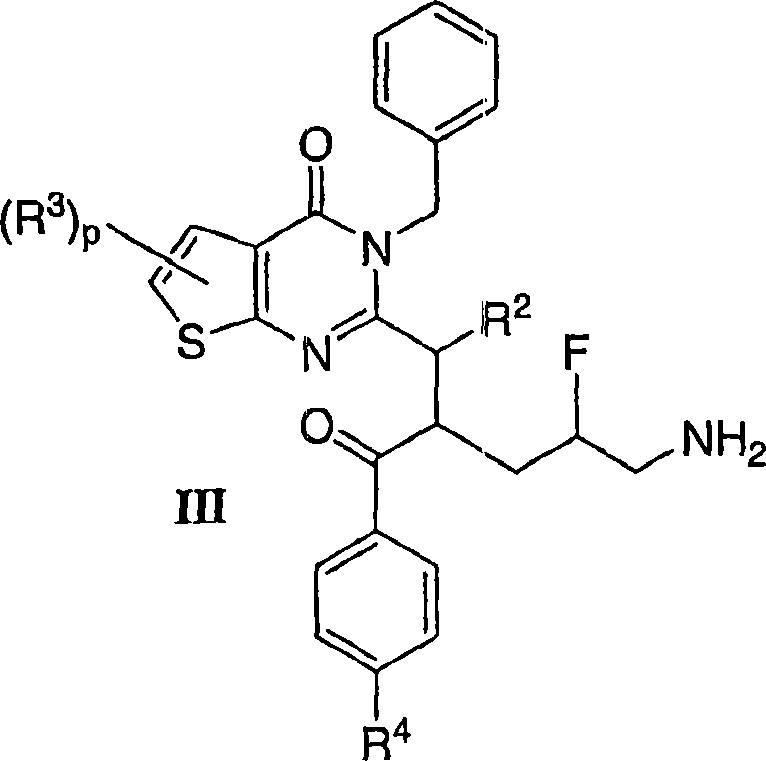
Mitotic kinesin inhibitors
An alkyl group selected technology, applied in the field of fluorinated 2-aminomethylthienopyrimidinone compounds, can solve problems such as limited applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0435] The examples provided are intended to assist in further understanding of the invention. The particular materials, substances and conditions employed are intended to be illustrative of the invention and not to limit the fair scope of the invention.
[0436] Flowchart 1
[0437]
[0438] Flowchart 1 (continued)
[0439]
[0440] Step 1: 2-[(3-Methylbutyryl)amino]thiophene-3-carboxylic acid (1-2)
[0441] 2-Aminothiophene-3-carboxylic acid methyl ester at 0°C 1-1 (5.0 g, 31.8 mmol) in DMF (30 mL) was added isovaleryl chloride (4.21 g, 34.9 mmol). The reaction was stirred at 0 °C for 2.5 h, then extracted with ether and washed with water. The organic solution was dried over sodium sulfate, filtered and concentrated to give the amide as an oil. To a solution of methyl 2-[(3-methylbutyryl)amino]thiophene-3-carboxylate (7.60 g, 31.49 mmol) in THF / MeOH was added 1 N KOH (2.65 g, 47.24 mmol) and the reaction was stirred overnight. The reaction was neutralized ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com