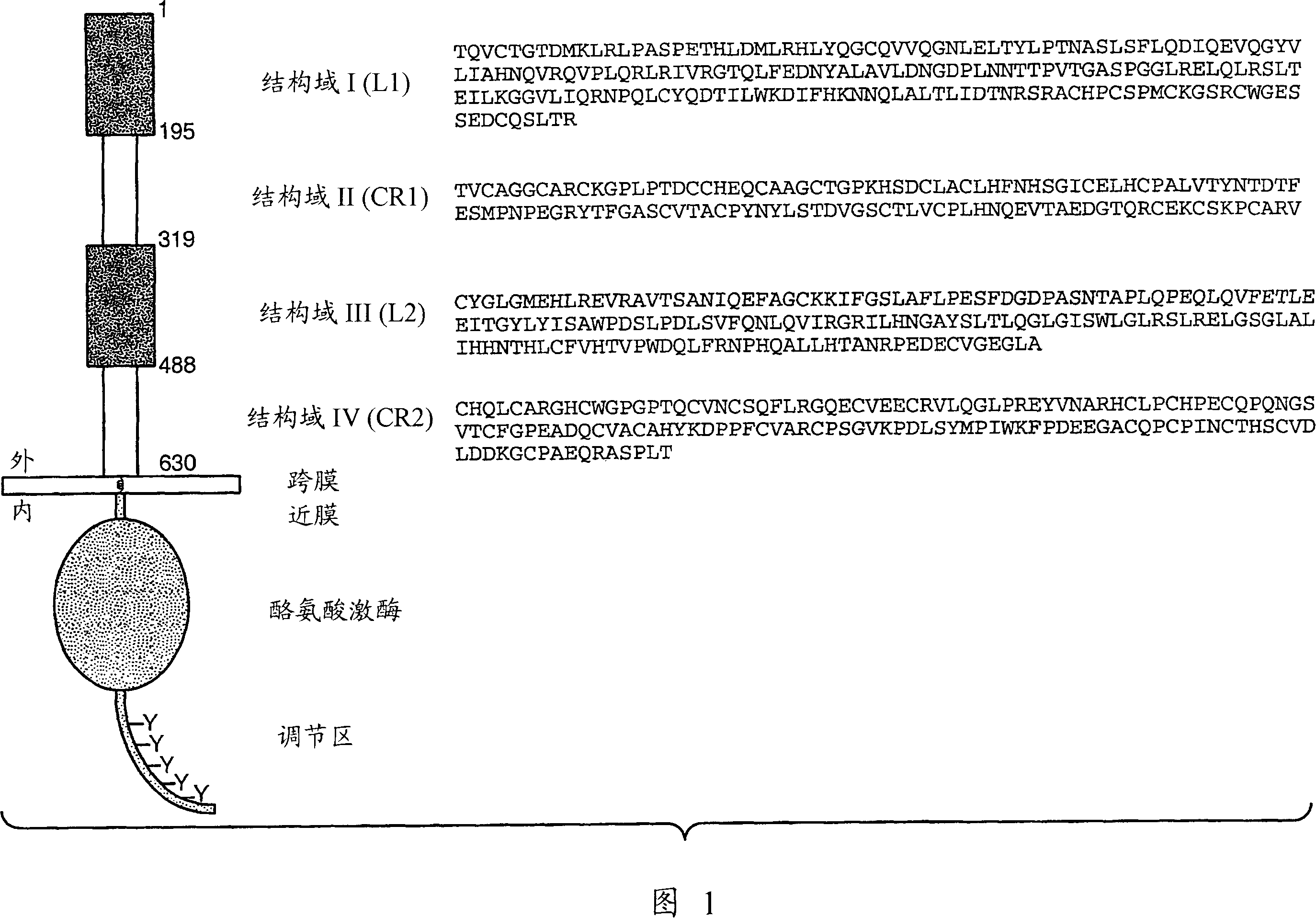
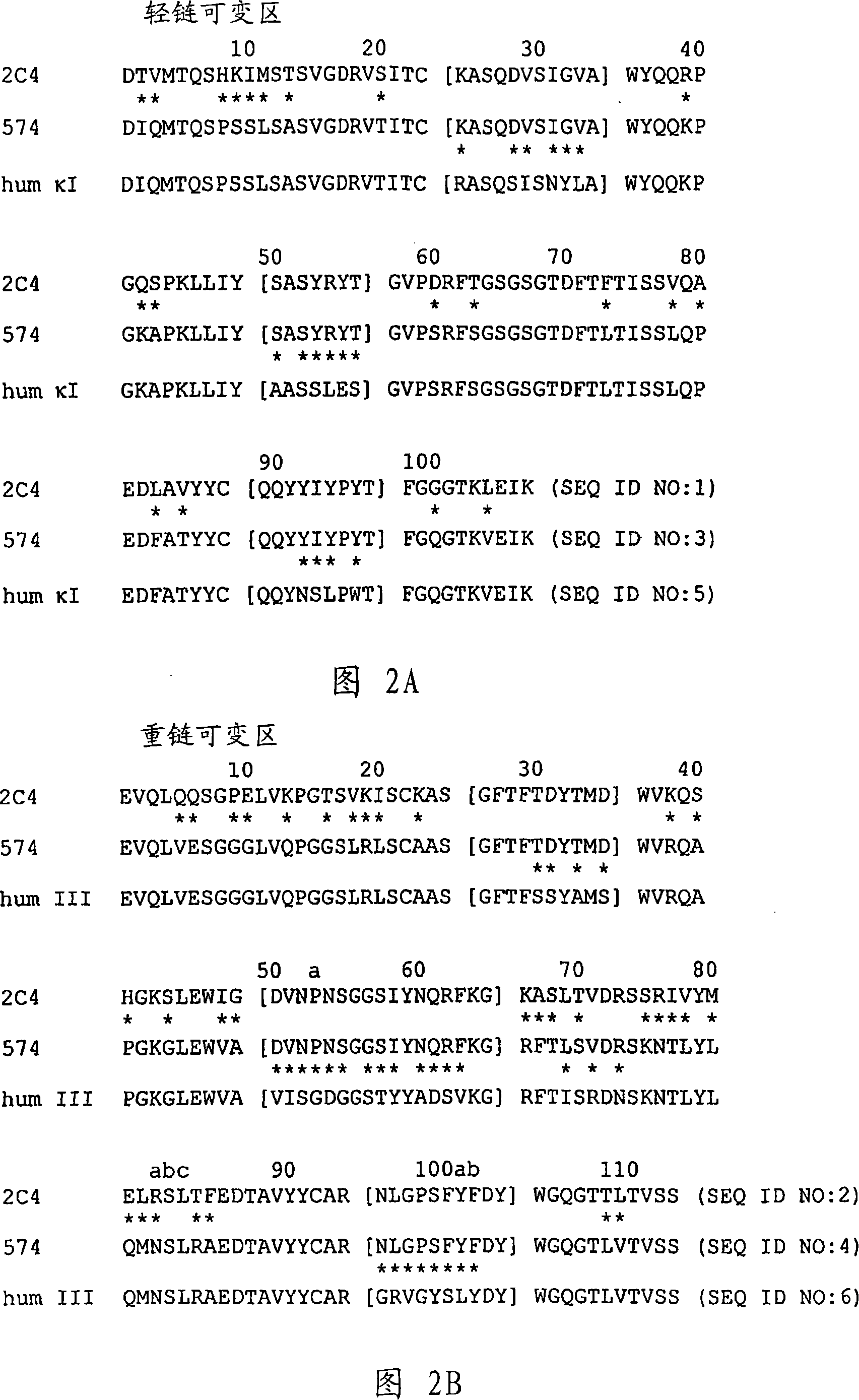
Selecting patients for therapy with a her inhibitor
An inhibitor, patient technology, applied in the direction of chemical instruments and methods, biochemical equipment and methods, and microbial assays/examinations, which can solve problems such as less favorable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Method used
Image
Examples
Embodiment 1
[0361] Example 1: Gene Expression Profiles Associated with HER2 Phosphorylation
[0362] Fresh ovarian tumor specimens from ovarian cancer patients treated with Pertuzumab were gene expression profiled for a small set of selected genes using AFFYMETRIX(R) microarray analysis. AFFYMETRIX(R) microarray analysis was performed according to the manufacturer's instructions.
[0363] Microarray expression data were analyzed to identify genotypes that would correlate with HER2 phosphorylation status. Notably, a pattern emerged in which tumors with relatively high levels of expression of EGFR, HER2, HER3, and the HER ligand betacelullin were also positive for HER2 phosphorylation. Figure 10 shows an unsupervised clustering of tumors with the above genes, resulting in clustering of 6 out of 6 phosphorylation positive tumors. Figure 11 shows that the phosphorylation status of ovarian tumors can be predicted by using an algorithm in which samples with betacellulin and HER2 expression at...
Embodiment 2
[0365] Example 2: Comparison of Microarray Analysis and qRT-PCR for Measuring Gene Expression
[0366] In this example, a second method for quantifying gene expression, quantitative real-time polymerase chain reaction (qRT-PCR), was used to validate and compare microarray data. qRT-PCT would be the preferred method for measuring gene expression in typical patient samples that can be obtained from a clinical setting. Diagnostic technology platforms for this approach have long been established.
[0367] qRT-PCR was performed as described in Cronin et al., Am. J. Pathol. 164(1):35-42 (2004) and Ma et al., Cancer Cell 5:607-616 (2004). RNA was extracted from frozen ovarian tumors using commercial reagents from Qiagen (Valencia, California). for TAQMAN TM Primers and probes for qRT-PCR analysis were designed to generate amplicons of approximately 100 bases or less in length. Use TAQMAN TM The facility (Applied BioSystems) quantified transcripts by qRT-PCR, normalizing the expr...
Embodiment 3
[0373] Example 3: Therapy for Patients with HER2 Phosphorylation-Associated Gene Expression Profiles
[0374] Examples 1 and 2 above demonstrate that gene expression profiling can be used as a surrogate for HER2 phosphorylation. In this example, patients whose tumors have a specific gene expression profile associated with HER2 phosphorylation are treated with the HER inhibitor Pertuzumab.
[0375] qRT-PCR was performed as described in Cronin et al., Am. J. Pathol. 164(1):35-42 (2004) and Ma et al., Cancer Cell 5:607-616 (2004). RNA was extracted from frozen ovarian tumors using commercial reagents from Qiagen (Valencia, California). for TAQMAN TM Primers and probes for qRT-PCR analysis were designed to generate amplicons of approximately 100 bases or less in length. Use TAQMAN TM The facility (Applied BioSystems) quantified transcripts by qRT-PCR, normalizing expression levels of test genes to reference genes.
[0376] An algorithm has been developed based on the gene exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com