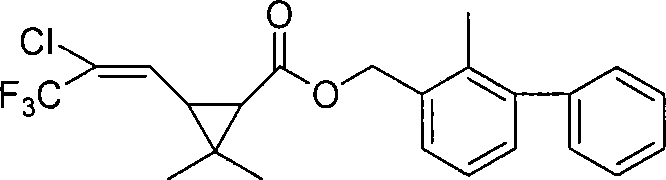
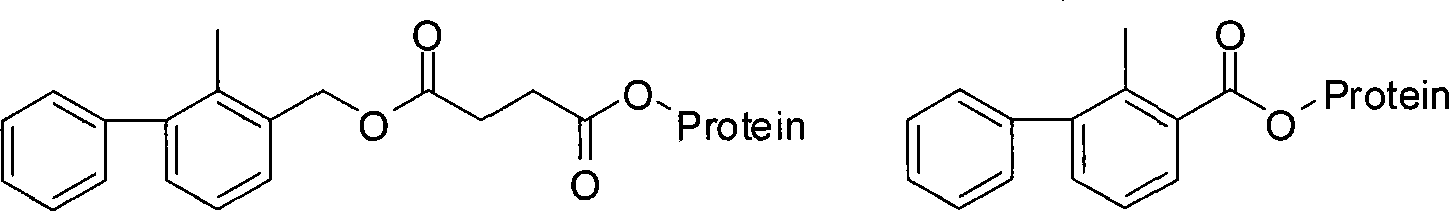Bifenthrin antigen, antibody and uses thereof
A technology of bifenthrin and antigen is applied in the field of immunochemical analysis to achieve the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] 1 Synthesis of Bifenthrin Artificial Hapten
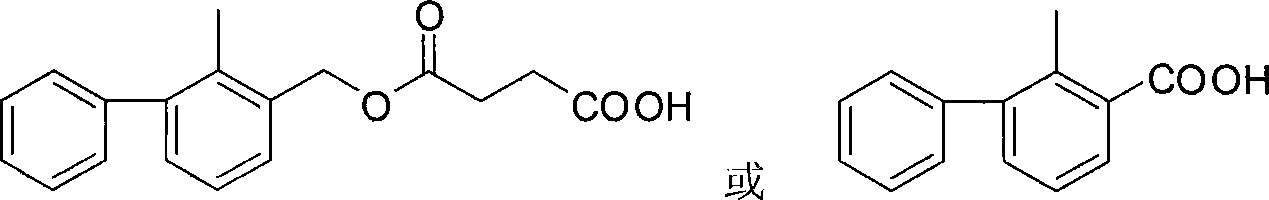
[0033] Based on the fact that 2-methyl-3-phenylbenzyl alcohol is the main component in the molecular structure of bifenthrin, and can fully expose the structural features and contain carboxyl groups that can be coupled with proteins, 2-methyl- Using 3-phenylbenzyl alcohol, succinic anhydride and anhydrous pyridine as raw materials, the hapten 2-methyl-3-phenylbenzylsuccinate (LBc for short) was synthesized through a one-step reaction. In order to improve the detection sensitivity through heterologous reaction, the hapten 2-methyl-3- Phenylbenzoic acid (abbreviated as LBy). After purification, the products LBc and LBy were subjected to mass spectrometry (ESI) and H NMR spectroscopy ( 1 H-NMR) identification, and confirmed by comparison with similar known compound data.
[0034] The structure of the hapten is as follows:
[0035]
[0036] 2-Methyl-3-phenylbenzylsuccinate (LBc) 2-Methyl-3-phenylbenzoic acid (LBy)
[003...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com