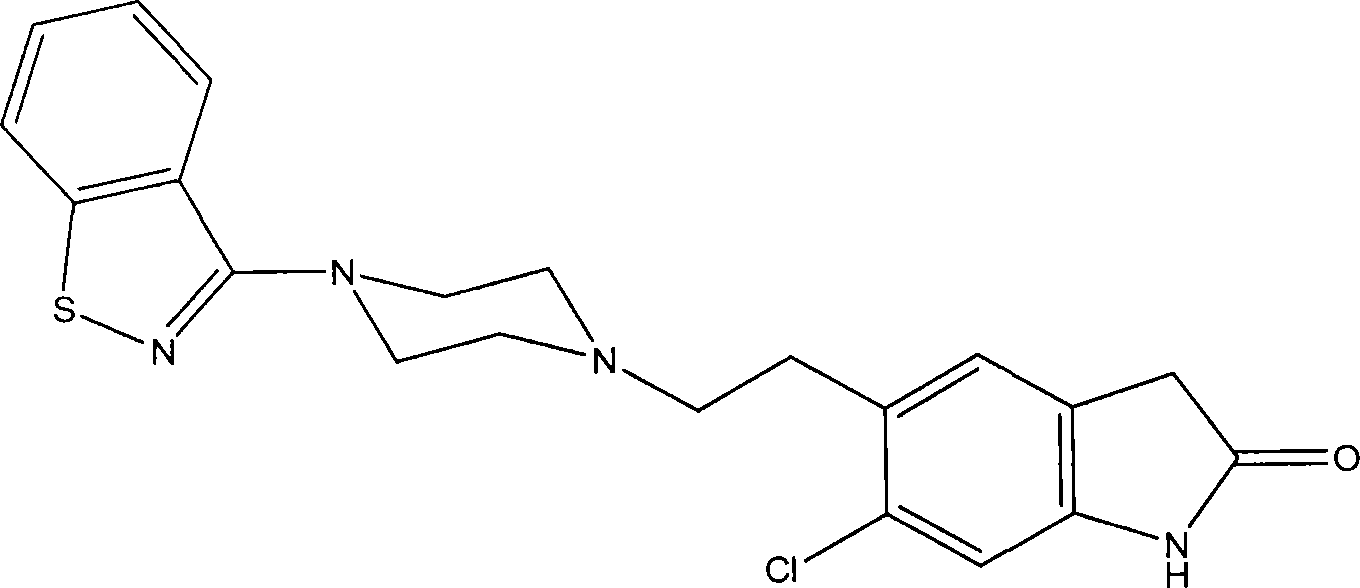
Injectable depot formulations and methods for providing sustained release of nanoparticle compositions
A nanoparticle and pharmaceutical preparation technology, applied in the field of ziprasidone for the treatment of mental illness, can solve the problems of poor water solubility of ziprasidone and difficulty in storage of ziprasidone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Preparation of Formulation A
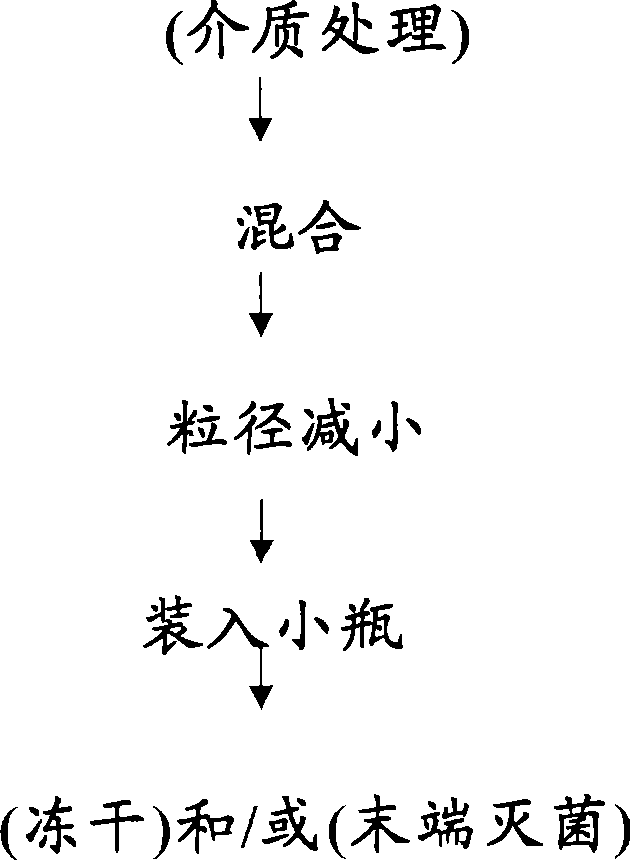
[0149] A crude suspension was prepared by placing 8.86 grams of ziprasidone free base and 48.90 grams of milling media (500 micron polystyrene beads) in a 100 ml milling chamber.
[0150] To this suspension, 4.2 ml each of 10% Pluronic(R) F108 solution and Tween(R) 80 solution were added. In addition, 27.8 ml of water for injection was added to the milling chamber. The above mixture is stirred until a homogeneous suspension is obtained. The suspension was then milled in Nanomill-1 (manufacturer: Elan Drug Delivery, Inc.) at 2100 RPM for 30 minutes, and the temperature was maintained at 4°C during the milling. The resulting suspension was filtered under vacuum to remove the milling media, and the suspension was characterized by microscopy and light scattering (Brookhaven). For microscopic observation, place a drop of the diluted suspension between the cover glass and the glass slide and observe in bright and dark fields. For light scattering p...
Embodiment 2
[0153] Preparation of Formulation B
[0154] A crude suspension was prepared by placing 8.84 grams of ziprasidone free base and 48.90 grams of milling media (500 micron polystyrene beads) in a 100 ml milling chamber.
[0155] To this suspension, 4.2 ml of a 10% solution of Pluronic(R) F108 was added. In addition, 32 ml of water for injection was added to the milling chamber. The above mixture was milled under the same conditions as in Example 1.
[0156] The milling was stopped at 30 minutes, at which time the above suspension became a paste, so a uniform, non-aggregated, free-flowing nano suspension was not obtained. Because the paste cannot be filtered to separate the milling media, no additional characterization can be performed.
Embodiment 3
[0158] Preparation of Formulation C
[0159] A crude suspension was prepared by placing 8.82 grams of ziprasidone free base and 48.87 grams of milling media (500 micron polystyrene beads) in a 100 ml milling chamber.
[0160] To this suspension, add 4.2 ml of a 10% solution of PVP-K30. In addition, 32 ml of water for injection was added to the milling chamber. The above mixture was milled under the same conditions as in Example 1.
[0161] The milling was stopped at 30 minutes, at which time the above suspension became a paste, so a uniform, non-aggregated, free-flowing nano suspension was not obtained. Because the paste cannot be filtered to separate the milling media, no additional characterization can be performed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com