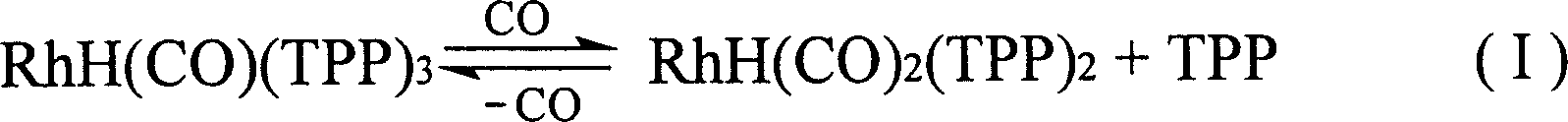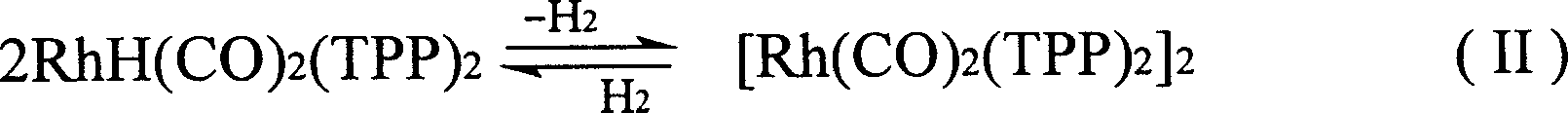Method for producing 3-(triphenylphosphine )-carbonyl hydrogenation Rh
A technology of rhodium carbonyl hydride and triphenylphosphine, which is applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problem of large consumption of raw materials such as solvents, complex processes, and overall The yield is not high, and the effect of good homogeneous catalytic activity for olefin hydroformylation is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] In the 500ml three-necked reaction flask, add 250ml mass percentage composition and be 95% ethanol aqueous solution, and 15.7 grams triphenylphosphine (TPP), after sufficient nitrogen replacement, under nitrogen protection (atmospheric pressure) stir and be heated to 80°C. After TPP is completely dissolved, add dropwise 10ml containing 1.77g RhCl 3 ·xH 2 O (the mass percentage of rhodium is 39.5%) ethanol solution. After staying for 30 minutes, 6ml of 40% formaldehyde aqueous solution was added dropwise, and the reaction temperature should be kept at 80°C during the addition. After adding the formaldehyde, the stirring reaction was continued for 60 minutes. Then, nitrogen was replaced by hydrogen (atmospheric pressure), and 30 ml of an ethanol solution containing 0.93 g of sodium borohydride and 1.4 g of KOH was added dropwise. After continuing to react at 80° C. for 60 minutes, the reaction was stopped and cooled to below 20° C. under a hydrogen atmosphere. The so...
Embodiment 2
[0052] In a 500ml three-necked reaction flask, add 250ml of 90% ethanol aqueous solution and 18.0 grams of triphenylphosphine. After sufficient hydrogen replacement, stir and heat to 80°C under hydrogen protection (atmospheric pressure). After TPP is completely dissolved, add dropwise 10ml containing 1.77g RhCl 3 ·xH 2 O (the mass percentage of rhodium is 39.5%) ethanol solution. After staying for 20 minutes, add 8 ml of 40% formaldehyde aqueous solution, and the reaction temperature should be kept at 80° C. during the addition. After the addition of formaldehyde, the stirring reaction was continued for 50 minutes. Then, 30 ml of an ethanol solution containing 1.13 g of sodium borohydride and 0.56 g of KOH was added dropwise. After continuing to react at 80°C for 50 minutes, the reaction was stopped and cooled to below 20°C under a hydrogen atmosphere. The solvent was removed by filtration under reduced pressure, washed with 95% ethanol, water, 95% ethanol, and n-hexane re...
Embodiment 3
[0054] In a 500ml three-necked reaction flask, add 250ml of 95% ethanol aqueous solution and 17.0 grams of triphenylphosphine. After sufficient hydrogen replacement, stir (normal pressure) under hydrogen protection and heat to 80°C. After TPP is completely dissolved, add dropwise 10ml containing 1.76g RhCl 3 ·xH 2 O (the mass percentage of rhodium is 39.5%) ethanol solution. After staying for 20 minutes, 11 ml of 40% formaldehyde aqueous solution was added, and the reaction temperature should be kept at 80° C. during the addition. After adding the formaldehyde, the stirring reaction was continued for 60 minutes. Then, 30 ml of an ethanol solution containing 1.50 g of sodium borohydride and 1.0 g of KOH was added dropwise. After continuing to react at 80° C. for 60 minutes, the reaction was stopped and cooled to below 20° C. under a hydrogen atmosphere. The solvent was removed by filtration under reduced pressure, washed with 95% ethanol, water, 95% ethanol, and n-hexane re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com