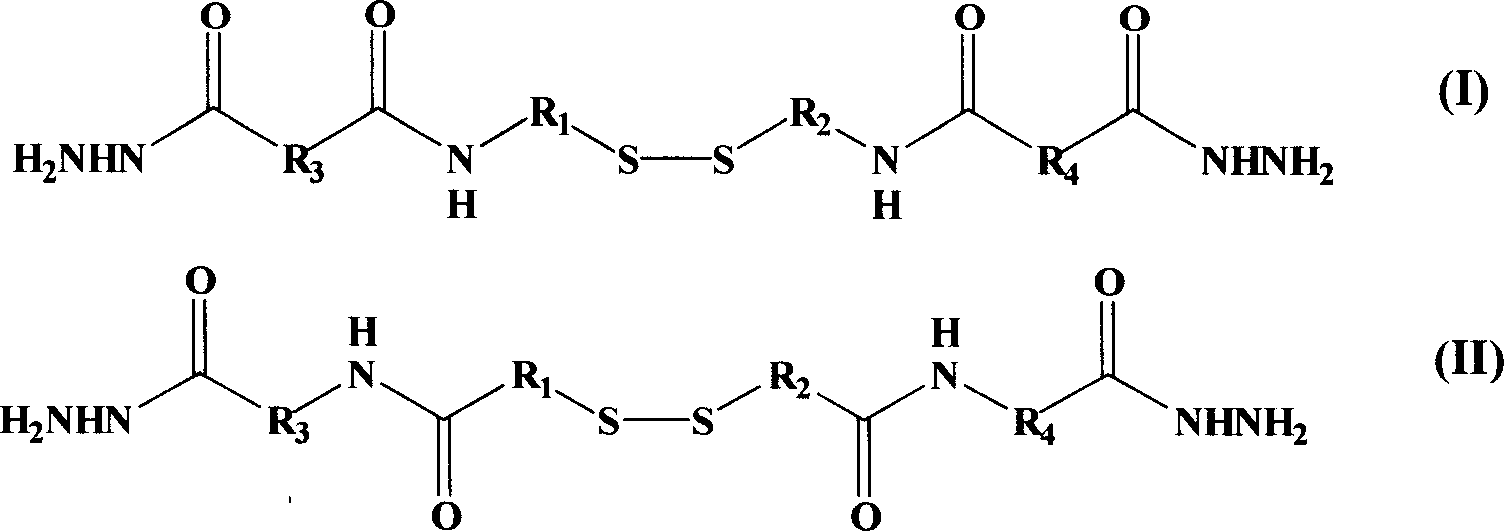
Dihydrazide compound and its preparation method and application
一种化合物、肼解反应的技术,应用在化合物领域,能够解决不能满足材料新型化学改性和化学交联等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
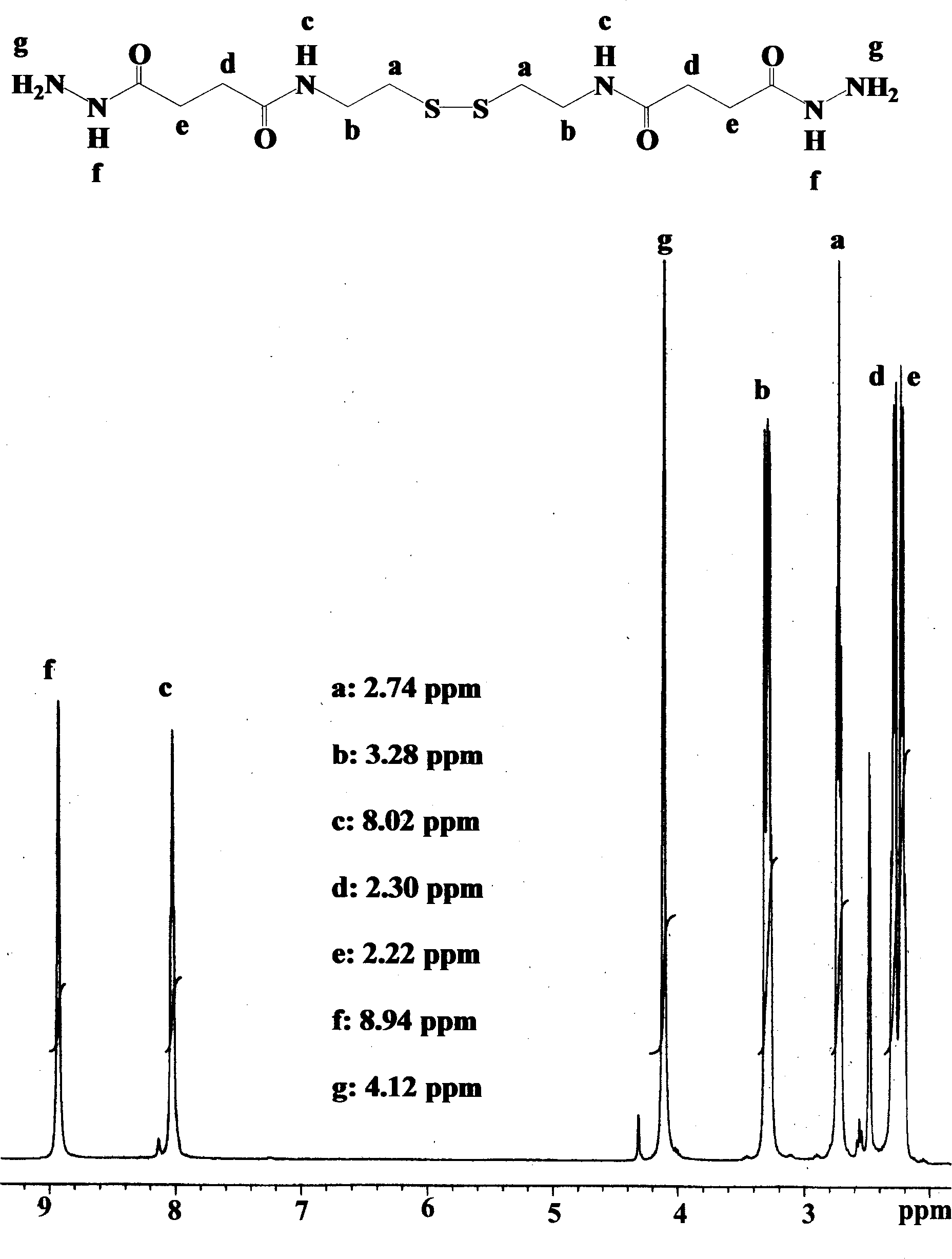
[0039] Preparation of disuccinic acid diacylcystine dihydrazide (DSCDH for short):
[0040] (1) Synthesis of disuccinic acid dicystine (abbreviated as DSC):
[0041] Cystamine dihydrochloride (Aldrich, USA) 10 g was dissolved in 150 ml distilled water to obtain a clear and transparent solution. Add 4mol / L sodium hydroxide to the above solution until the pH value of the solution is 10. Then, 13.3 g of succinic anhydride (Aldrich, USA) was added under electromagnetic stirring, and at the same time, 4 mol / L sodium hydroxide was continuously added to keep the pH value of the solution at 7-10. After reacting at room temperature for 2 hours, 6 mol / L hydrochloric acid was added to the solution. The white precipitated product was collected by filtration and washed twice with 200 ml of distilled water. Then it was dried under reduced pressure in vacuo to obtain about 15 g of white product DSC as a solid product, and the yield was greater than 90%.
[0042] (2) Synthesis of disuccinic...
Embodiment 2
[0052] Preparation of disuccinic acid diacylcystine dihydrazide (DSCDH for short):
[0053] (1) Synthesis of disuccinic acid dicystine (abbreviated as DSC): same as Example 1.
[0054] (2) Synthesis of activated bisuccinic acid bisacylcystine biscarbonyldiimidazole ester (DSCDI for short):
[0055] Add 10g DSC, 60ml anhydrous DMF in the 500ml three-neck round bottom flask, after stirring and dissolving at room temperature, add 11.2g carbonyldiimidazole (Aldrich, the United States), at this moment, the solution produces a large amount of carbon dioxide bubbles and white precipitate, and the reaction under reduced pressure at room temperature is 3 hours; then add 200ml of anhydrous ethyl acetate to dilute, and collect the precipitated product by filtration. The precipitate was rinsed twice with 200 ml of anhydrous ethyl acetate, and then dried under reduced pressure in vacuo to obtain about 12 g of white solid product DSCDI, with a yield greater than 90%.
[0056] (3) Synthesi...
Embodiment 3
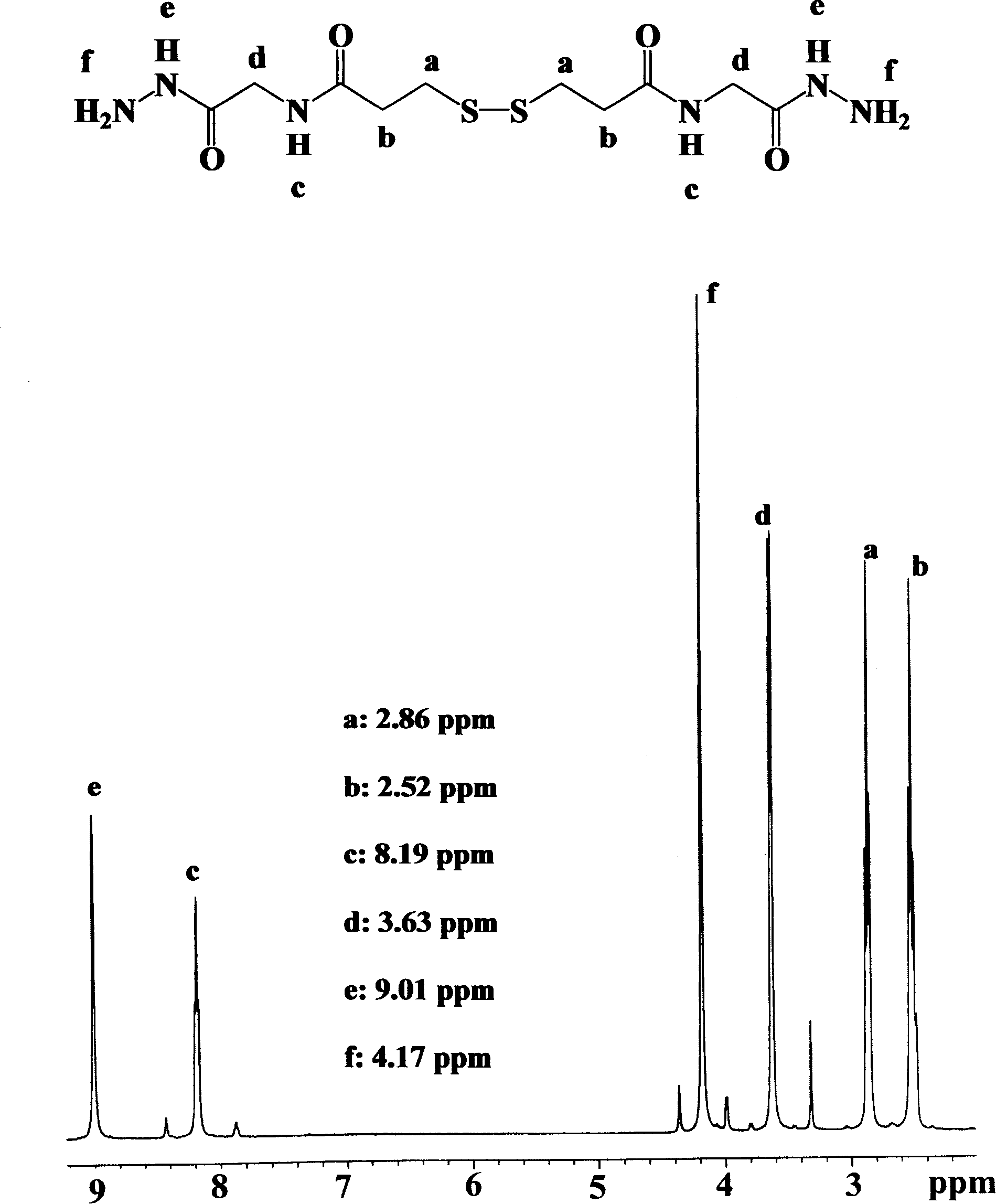
[0059] Preparation of Diglutaric Acid Diacylcystine Dihydrazide (abbreviated as DGCDH):
[0060] (1) Synthesis of double glutaric acid dicystine (abbreviated as DGC):
[0061] Cystamine dihydrochloride (Aldrich, USA) 10 g was dissolved in 150 ml distilled water to obtain a clear and transparent solution. Add 4mol / L sodium hydroxide to the above solution until the pH value of the solution is 10. Then, 15.2 g of glutaric anhydride (Aldrich, USA) was added under electromagnetic stirring, and at the same time, 4 mol / L of sodium hydroxide was continuously added to keep the pH value of the solution at 7-10. After reacting at room temperature for 2 hours, 6 mol / L hydrochloric acid was added to the solution. The white precipitated product was collected by filtration and washed twice with 200 ml of distilled water. Then it was dried under reduced pressure in vacuo to obtain about 15.5 g of white solid product DGC, and the yield was greater than 90%.
[0062] (2) Synthesis of diethy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com