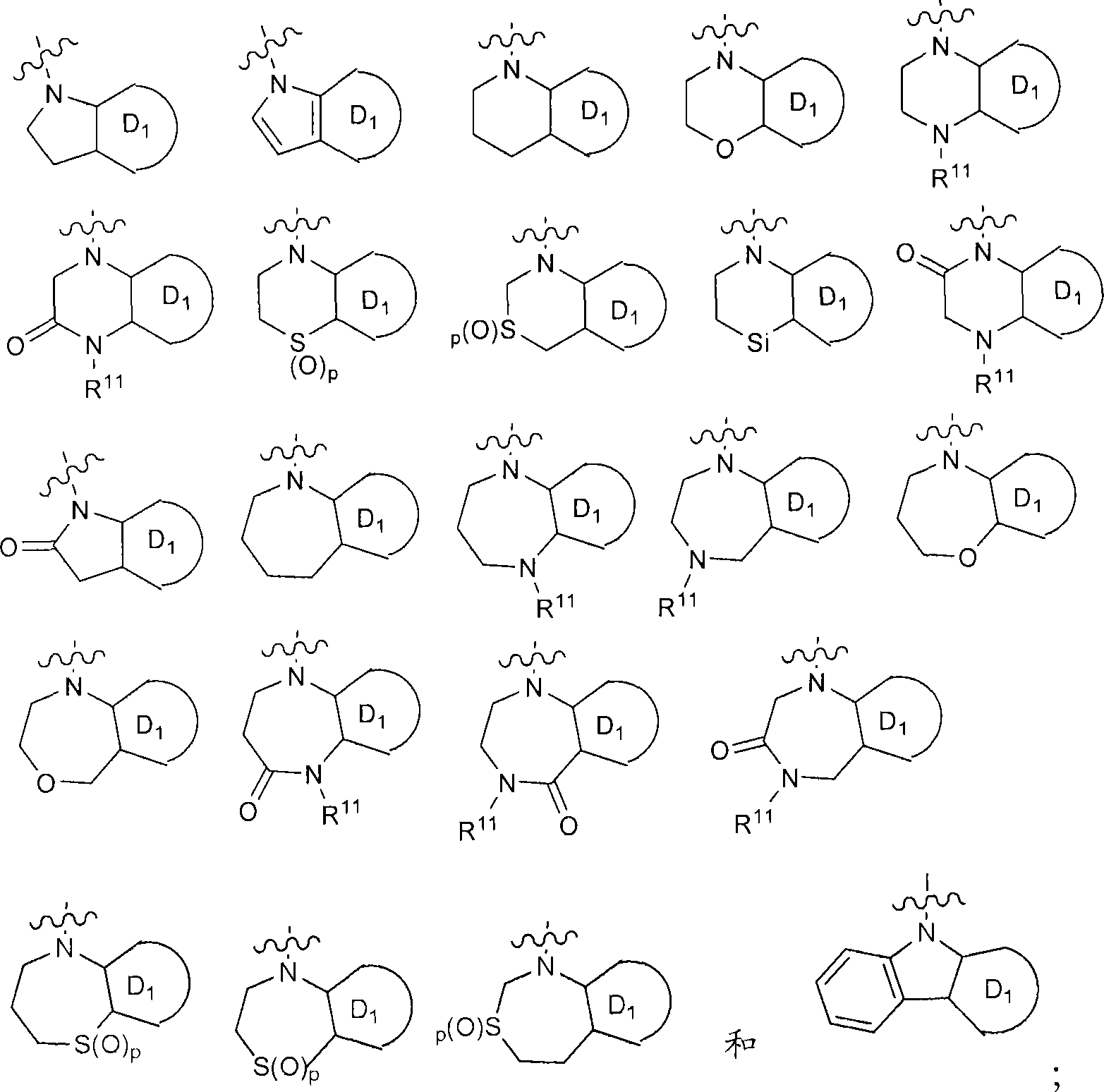N-linked heterocyclic antagonists of P2Y1 receptor useful in the treatment of thrombotic conditions
A heterocyclic and carbocyclic technology, applied in the field of N-linked heterocyclic antagonists of P2Y1 receptors for the treatment of thrombotic symptoms, can solve the lack of pharmacological properties and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
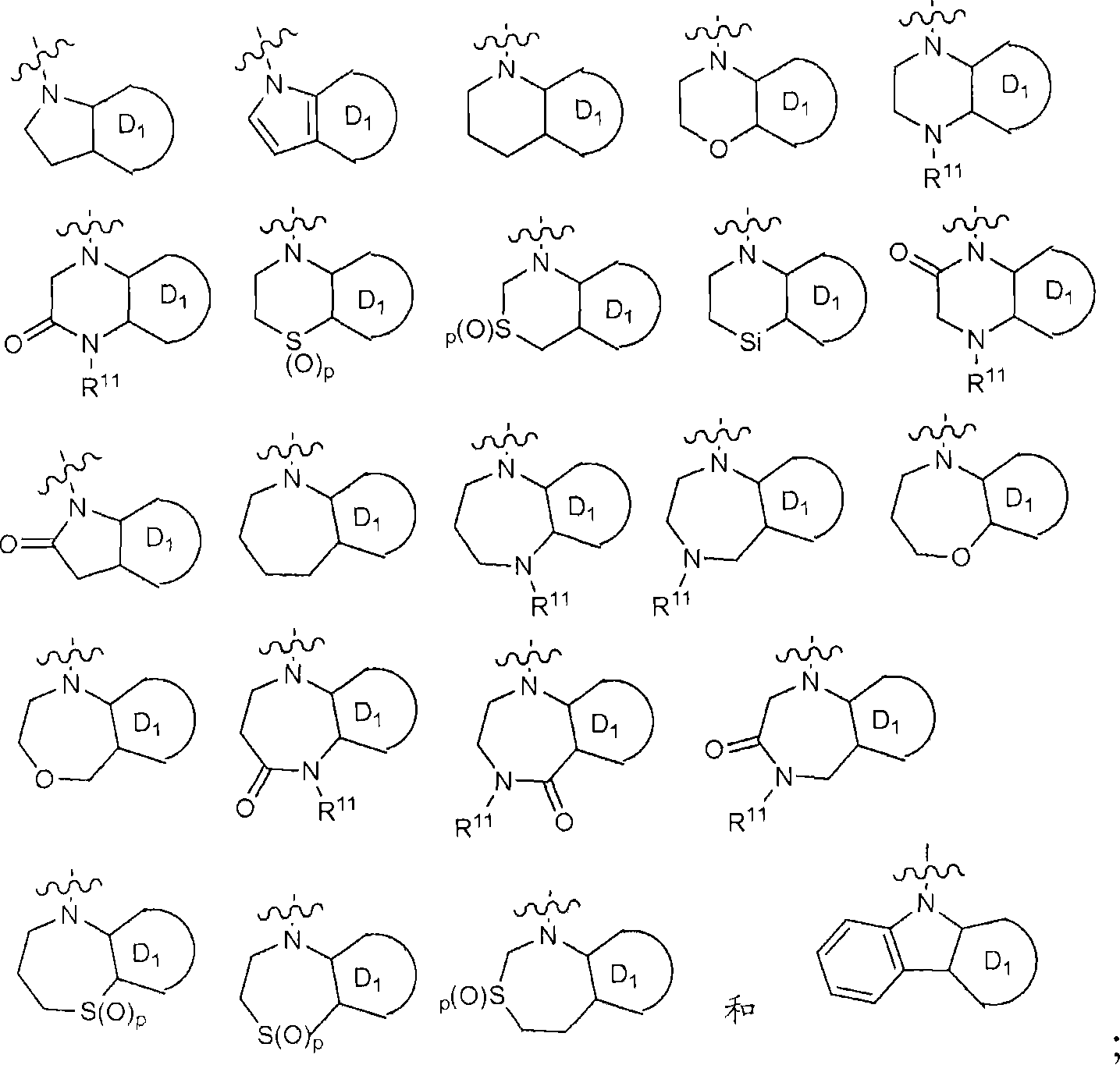
[0463] Scheme 6 describes the preparation of compounds of the invention wherein the A ring is derived from a functionalized intermediate of formula 6.1 wherein Z is nitrogen or sulfur. Intermediate 6.1 is treated with reagents such as α-haloketones in solvents such as ethanol with or without a base such as 2,6-lutidine or NaOAc at temperatures from 0°C to 110°C Or α-haloaldehydes or equivalent reagents treat the intermediates to give compounds of formula 6.3 (for analogous chemistry with Z=sulfur see: Udapudi, V.T. et al. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry 1986, 25B(12), 1269-72. Singh, S.P.; et.al. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry 1985, 24B(1), 119-23).
[0464] Option 6
[0465]
[0466] Scheme 7 describes the preparation of compounds of the invention wherein the A ring is a substituted oxazole from a functionalized intermediate of formula 7.1. Intermediate 7.1 is...
Embodiment 1
[0553] N-(2-(4,4-Dimethyl-3,4-dihydroquinolin-1(2H)-yl)phenyl)-4-(trifluoromethyl)thiazol-2-amine
[0554]
[0555] 1a. 4,4-Dimethyl-3,4-dihydro-1H-quinolin-2-one:
[0556] Aniline (7.26 g, 45.2 mmol) and 3-methylbut-2-enoyl chloride (53.2 g, 45.2 mmol) were heated to reflux in chloroform for 2 hours. After cooling, the mixture was filtered. The filtrate was concentrated to dryness and dried in vacuo. Crude 3-methyl-but-2-enoic acid phenylamide (about 10 g) was dissolved in toluene (50 mL). This toluene solution was added portionwise to the stirred AlCl 3powder (27g). After the addition, the resulting dark brown solution was heated at 80 °C for 2.5 hours. The warm slurry was carefully poured over stirred crushed ice. The resulting mixture was extracted with EtOAc (3x), washed with saturated NaHCO 3 、H 2 O and brine washed and washed with MgSO 4 dry. The residue was subjected to silica gel flash chromatography (CH 2 Cl 2 , followed by EtOAc) to yield 2.2 g of p...
Embodiment 2
[0572] N-(2-(3,3-Dimethylindolin-1-yl)phenyl)-4-(trifluoromethyl)thiazol-2-amine
[0573]
[0574] 2a. 1-Acetyl-1,3-dihydro-indol-2-one:
[0575] Indolin-2-one (6.65 g, 50 mmol) and acetic anhydride (9 mL) were heated to reflux for 15 hours. After cooling, the product was filtered and washed with Et 2 After washing with O and drying in vacuo, 2a (8.2 g, 93.7% yield) was obtained as a solid.
[0576] 2b. 3,3-Dimethylindolin-2-one:
[0577]
[0578] To a stirred solution of 2a (3.2 g, 18.29 mmol) in anhydrous THF (100 mL) was added MeI (2.6 mL, 41.75 mmol, 2.3 eq) followed by 18-crown-6 ( 0.51 g, 4.57 mmol, 0.25 eq). Potassium tert-butoxide (5.12 g, 45.73 mmol, 2.5 eq) was added in portions. The resulting slurry was stirred at -78°C for 1 hour. The mixture was stirred at -78°C to room temperature for 3 hours. Cool in an ice bath, add saturated NH 4 Cl. It was extracted with EtOAc, washed with H 2 O and brine washed with MgSO 4 Dry, filter, and concentrate to d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com