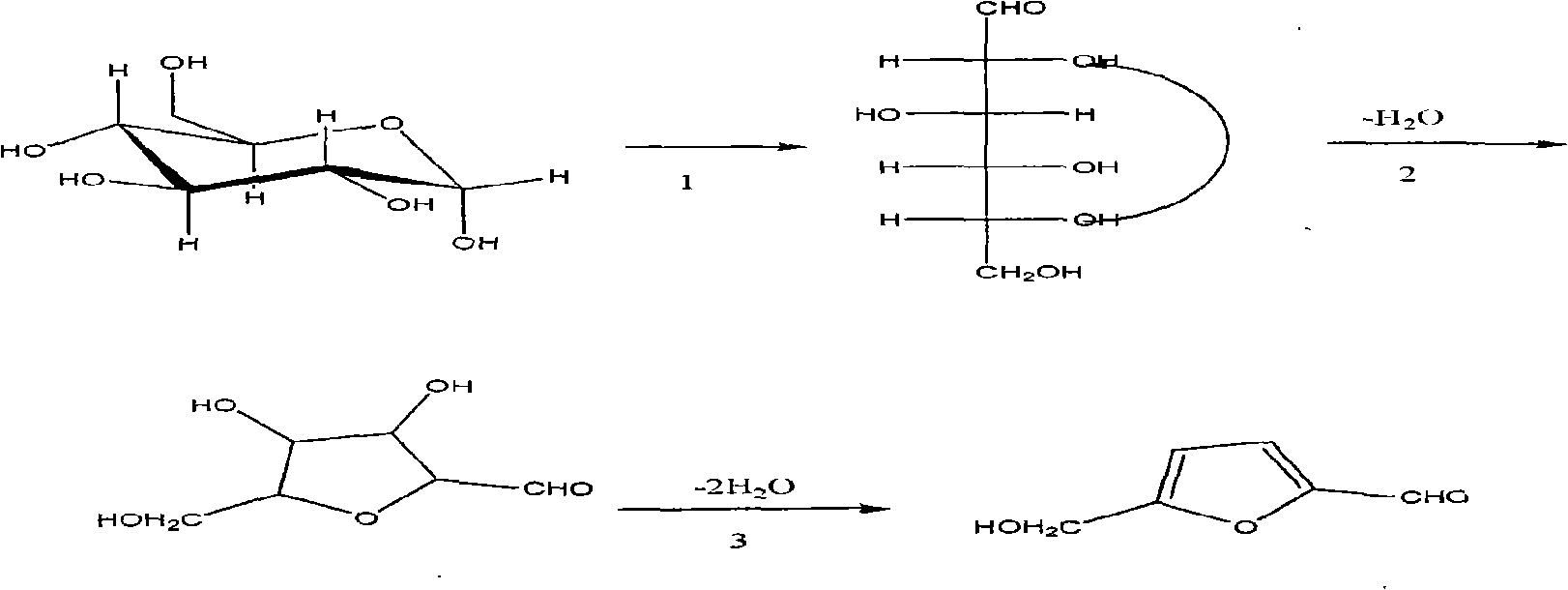
Method for synthesizing 5-hydroxymethyl-furfural
A technology of hydroxymethylfurfural and synthetic method, which is applied in the field of bioorganic synthesis of 5-hydroxymethylfurfural, and can solve problems such as complicated processing, high price, and limited use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Add 40g of chromium chloride and 160g of caprolactin to the flask, heat to increase the temperature (before 60°C, increase the temperature by 20°C per hour, and then increase the temperature by 10°C per hour) to melt at 120°C to become a uniform ionic liquid. The configured ionic liquid was slowly cooled to below 80°C, and 45 g of glucose (pharmaceutical grade) was added in batches, while stirring. After adding and dissolving evenly, slowly increase the temperature and depressurize (control pressure 0.05~0.07MPa) for dehydration. When the temperature rises above 100°C, keep the temperature, continue the reaction and dehydration until no water volatilizes to obtain the post-reaction The mixture of ionic liquid and dark gray liquid together.
[0013] The reaction mixture is transferred to a set of vacuum rectification device while it is hot, and the system pressure is maintained at 150-180KPa and the temperature is 90-105°C, and a certain material transfer rate is controlled ...
Embodiment 2
[0016] Add 50g of chromium chloride and 200g of caprolactin to the flask, heat to increase the temperature (before 60°C, increase the temperature by 20°C per hour, and then increase the temperature by 10°C per hour) to melt at 120°C to become a uniform ionic liquid. The configured ionic liquid was slowly cooled to below 80°C, and 60 g of glucose (pharmaceutical grade) was added in batches, while stirring. After adding and dissolving uniformly, slowly increase the temperature and depressurize (control pressure 0.05~0.06MPa) for dehydration. When the temperature is above 100℃, keep the temperature, continue the reaction and dehydration until no water volatilizes, and obtain the reacted Dark gray liquid with mixture and ionic liquid.
[0017] Transfer the reaction mixture to a set of vacuum rectification device while it is hot, and keep the system pressure 150~160KPa and temperature 95~103
[0018] ℃, control a certain material transfer speed, so that products, by-products and remain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com