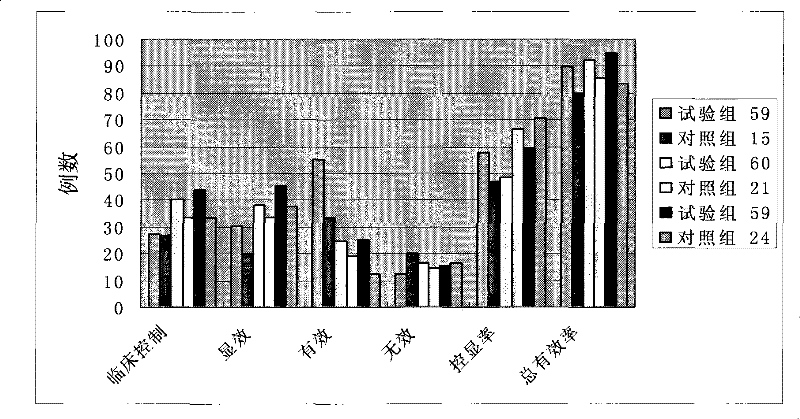
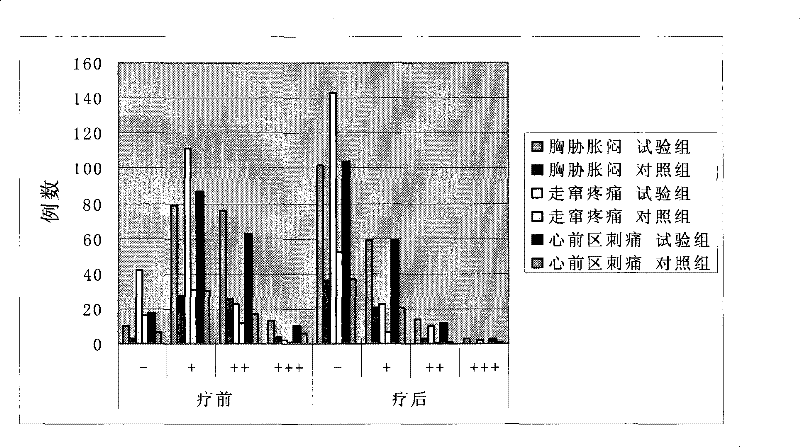
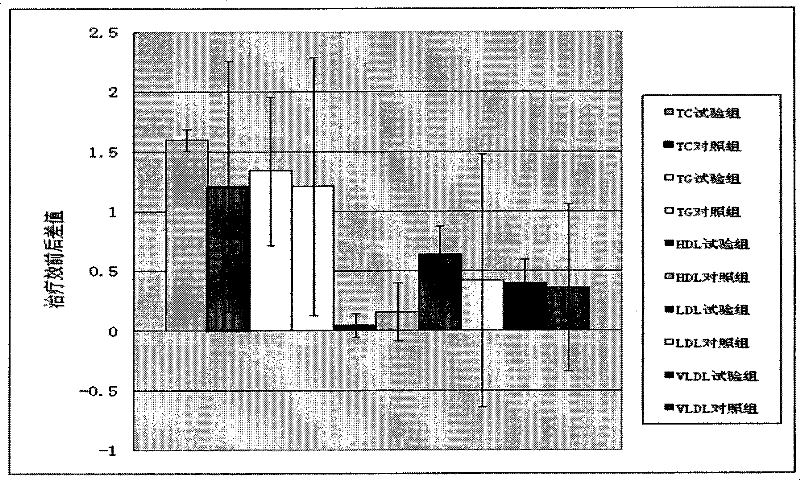
Process for extracting dioscin, preparation thereof and use
A technology of diosgenin and extract, which is applied in the field of preparation of drugs for the prevention and treatment of cardiovascular diseases, can solve the problems of uneven coating dispersion, slow drug effect, and affecting drug properties, and achieves low one-time investment cost and easy industrialization The effect of mass production and improvement of clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Comparative experiment on the preparation of dioscin by multifunctional scouring enzyme DMA enzymatic hydrolysis and weak polar macroporous resin extraction method (referred to as compound extraction method) and enzymatic hydrolysis method
[0045] Experiment 1: Take 25g of dried Dioscorea scutellaria powder, add quantitative water to a three-necked flask, stir evenly, add 0.1% cellulase and pectinase compound enzyme preparation at a temperature of 50°C for 12 hours, and then add a certain amount of Concentrated H 2 SO 4 Prepare 2 mol / L hydrolyzate at 100°C for 4 hours, filter, wash the filter residue with 10% NaOH solution until neutral, dry, and grind to obtain a powder extract. After the extract was reflux extracted for 8 hours with a Soxhlet extractor, the extract was concentrated with a rotary evaporator and the product was crystallized, and finally dried in a vacuum oven at 80-100°C to obtain dioscin, and the yield and melting point of the obtained product were d...
Embodiment 2
[0051] Take 2kg of dry Dioscorea scutellaria powder, add a certain amount of water, stir evenly, add 30g / L of multifunctional scouring enzyme DMA dissolved in 30°C water, then add cold water to adjust the water amount, add the required 8g / L hydrogen peroxide and wash agent, stir evenly, heat up to 70°C and keep warm for 50min to obtain the extract;
[0052] Add 6 times the amount of water to the obtained extract, heat and reflux to extract twice, each time for 1 hour, filter, combine the extracts, and concentrate to 0.25g crude drug / ml; after filtering, pass through AB-8 macroporous resin, and extract the extract according to dioscin The ratio of the content meter to the dry resin is 1:5, the column flow rate is 1BV / h, and the diameter of the resin is 1:3; the resin is washed with water for 3BV, and the elution flow rate is 2BV / h, and the water eluent is discarded; % water-containing ethanol to elute 8BV, the flow rate is 2BV / h, and the ethanol eluate is collected; the ethanol...
Embodiment 3
[0054] Take 1kg of dry Dioscorea baicalensis powder, add a certain amount of water, stir evenly, add multifunctional scouring enzyme DMA15g / L dissolved in 60°C water, then add cold water to adjust the water amount, add the required 10g / L hydrogen peroxide and wash agent, stir evenly, and heat up to 80°C for 60 minutes to obtain the extract;
[0055] Add 15 times the amount of 30% ethanol to the obtained extract, heat and reflux to extract 4 times, each time for 3 hours, filter, combine the extracts, and concentrate to 2.0g crude drug / ml; The ratio of the diosgenin content meter to the dry resin is 1:10, the upper column flow rate is 4BV / h, and the diameter of the resin is 1:9; the resin is washed with water for 8BV, and the elution flow rate is 4BV / h, and the water eluent is discarded; Contain 95% aqueous ethanol for elution, flow rate is 4BV / h, collect ethanol eluate; ethanol eluate is concentrated under reduced pressure to a relative density of 1.25, and dried under reduced ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com