Pentaerythritols chiral spiro compound and synthesis and resolution method thereof
A technology of spiro compound and pentaerythritol, which is applied in the field of synthesis and resolution of spiro compound, can solve the problems of complex post-processing, corrosion equipment, and many side reactions, and achieve the effects of low cost, increased safety, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
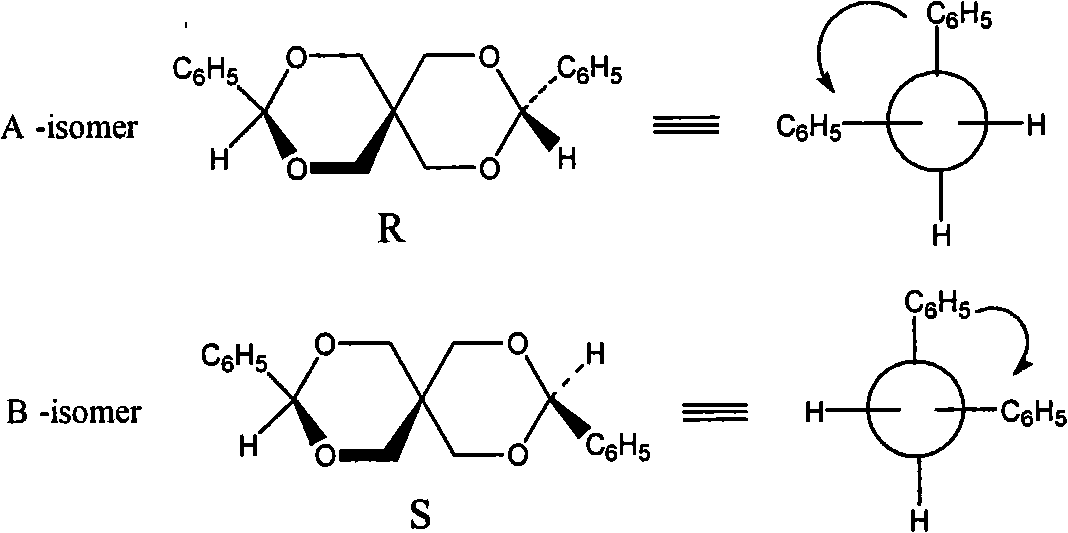
[0034] Embodiment 1: A kind of pentaerythritol chiral spiro compound, its name and structural formula are as follows:
[0035]
[0036] 3,9-bis(4-formylphenyl)-2,4,8,10-tetraoxa-spiro[5.5]-undecane,
[0037] The raw materials it adopts are substituted benzaldehyde and pentaerythritol, and the specific reaction equation is as follows:
[0038]
[0039] The specific synthesis steps are: take the substituted benzaldehyde and pentaerythritol in a ratio of 1:5, put the pentaerythritol into 120 mL of water, dissolve the substituted benzaldehyde in a small amount of ethanol, select 8 mL of HCl as the catalyst, and stir it mechanically for 8 hours. Filter, dry, and recrystallize with ethanol to obtain a light yellow solid.
[0040] Wherein the synthetic raw material of substituted benzaldehyde is terephthalaldehyde and acetic anhydride, and concrete reaction equation is as follows:
[0041]
[0042] The specific synthesis steps are as follows: dissolve terephthalaldehyde an...
Embodiment 2
[0049] Embodiment 2: a kind of pentaerythritol chiral spiro compound, its name and structural formula are as follows:
[0050]
[0051] 3,9-Diphenyl-2,4,8,10-tetraoxa-spiro[5.5]-undecane,
[0052] The raw materials it uses are benzaldehyde and pentaerythritol. The specific reaction equation is as follows:
[0053]
[0054] The specific synthesis includes the following steps: put benzaldehyde and pentaerythritol in a ratio of 2:1 into a three-necked flask containing 120 mL, pour 15 mL of HCl as a catalyst into the three-necked flask, stir at room temperature for 8 hours, filter, and The filter cake was recrystallized to give 3,9-diphenyl-2,4,8,10-tetraoxa-spiro[5.5]-undecane as white crystals.
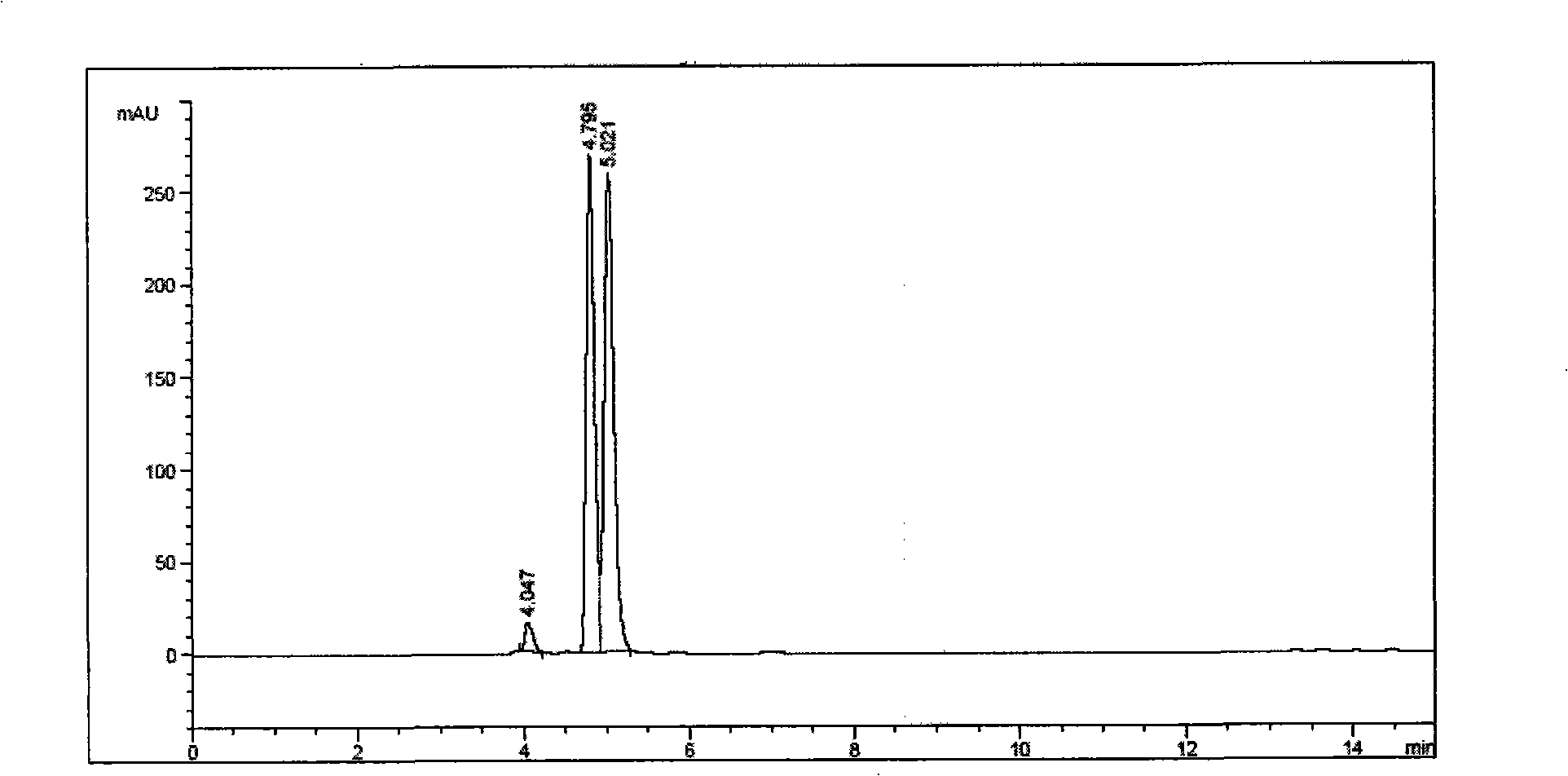
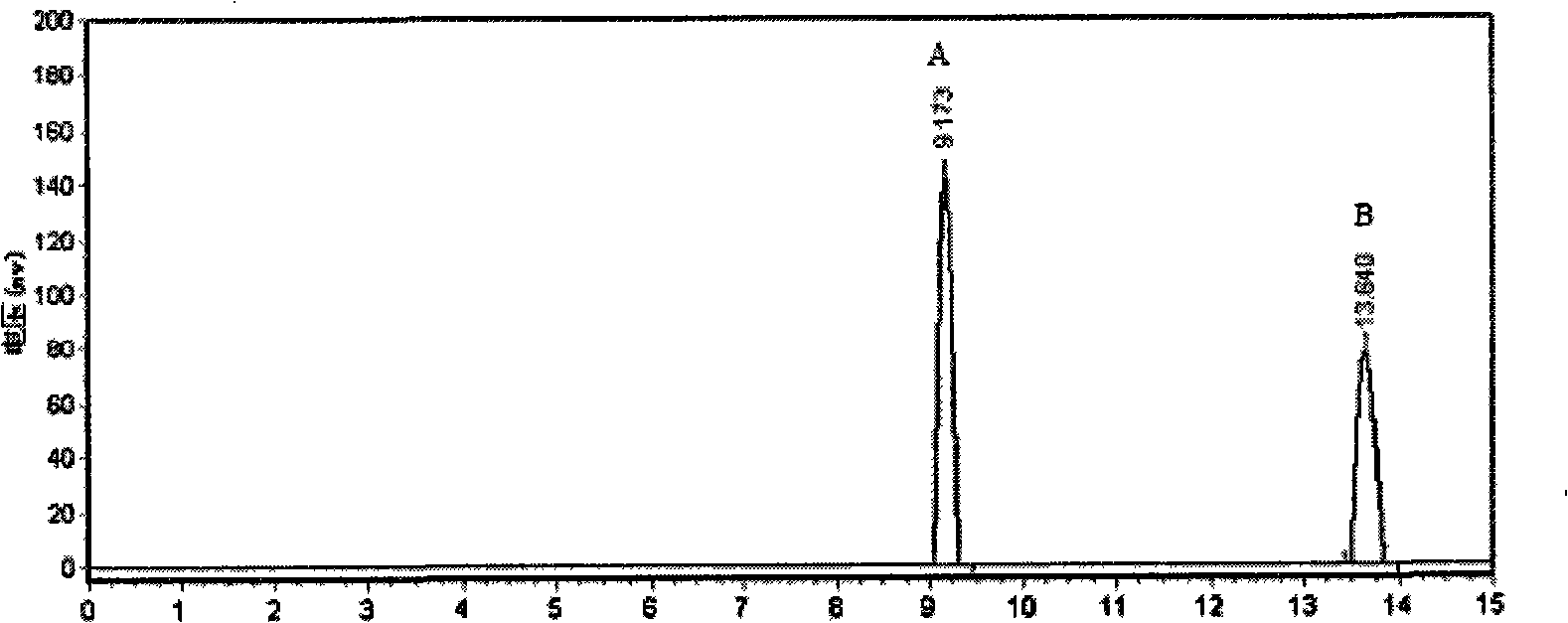
[0055] The resolution method of 3,9-diphenyl-2,4,8,10-tetraoxa-spiro[5.5]-undecane comprises the following steps:
[0056] (1) Dissolve 0.2 g of 2,9-diphenyl-2,4,8,10-tetraoxa-spiro[5.5]-undecane in the mobile phase as n-hexane:isopropanol=98:2 middle;
[0057] (2) Using amylo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com