Process and apparatus for the combustion of sulfur
A kind of equipment, air combustion technology, applied in the direction of chemical instruments and methods, sulfur compounds, inorganic chemistry, etc., can solve the problems of expensive, multi-level equipment complex price and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
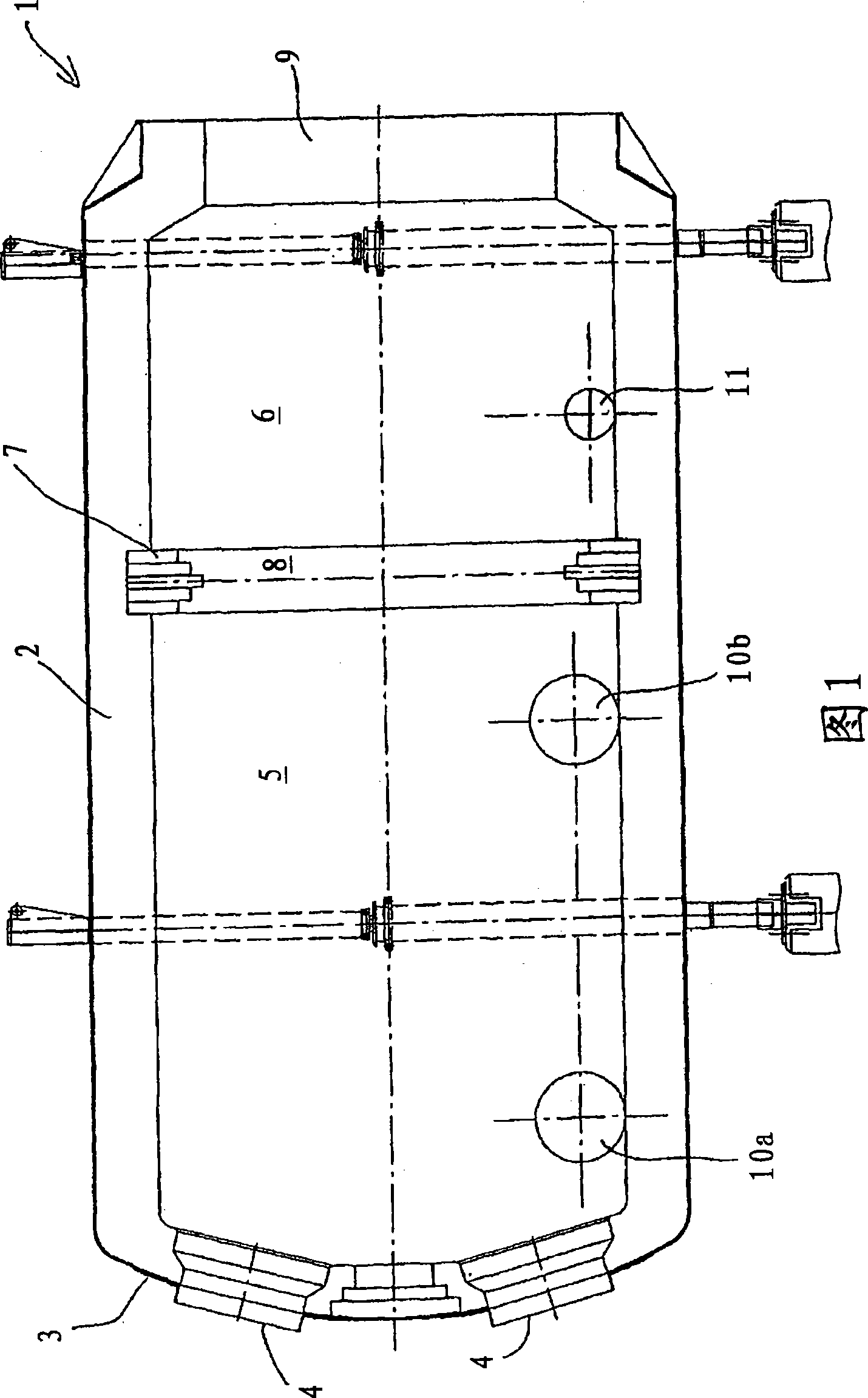
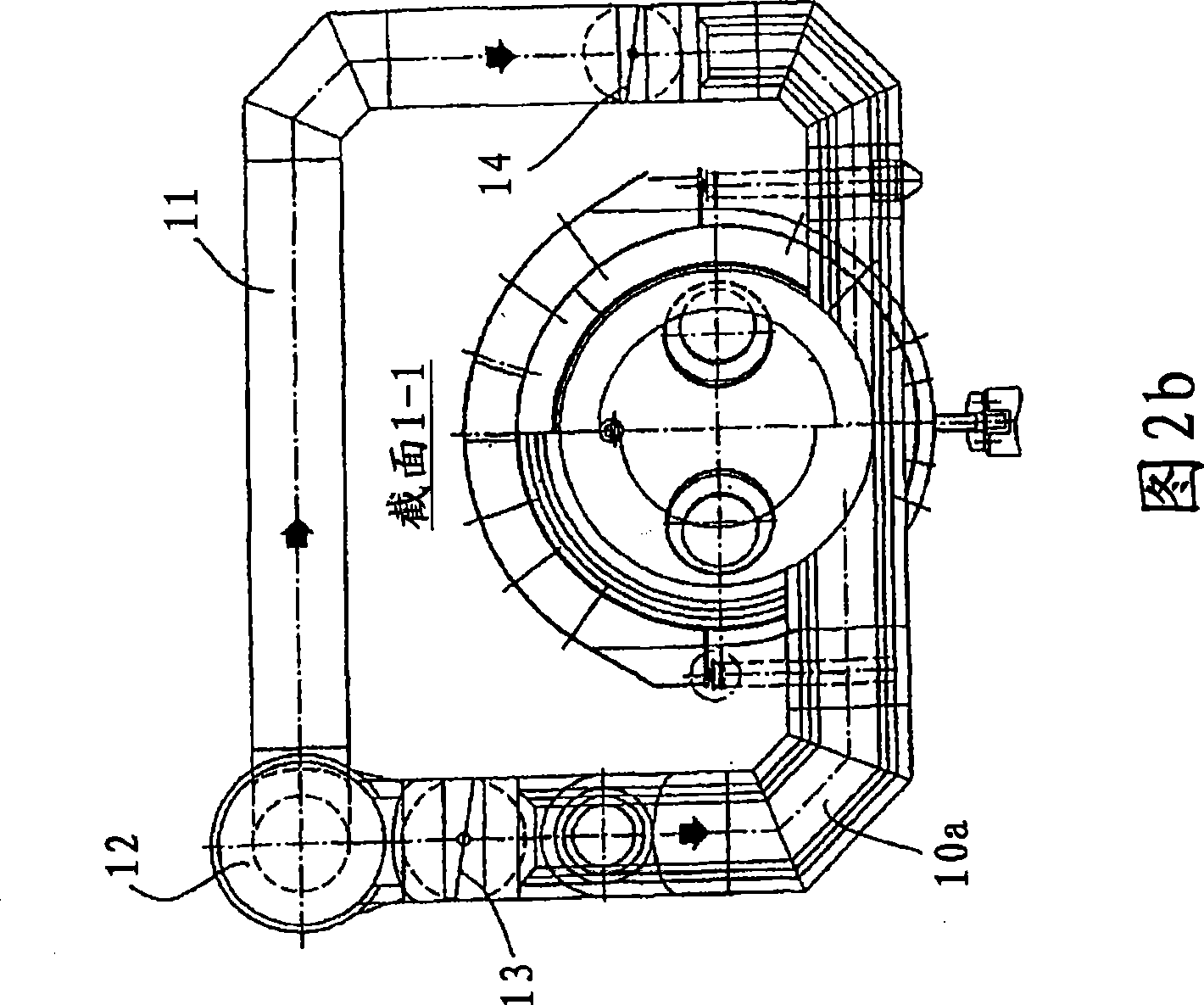
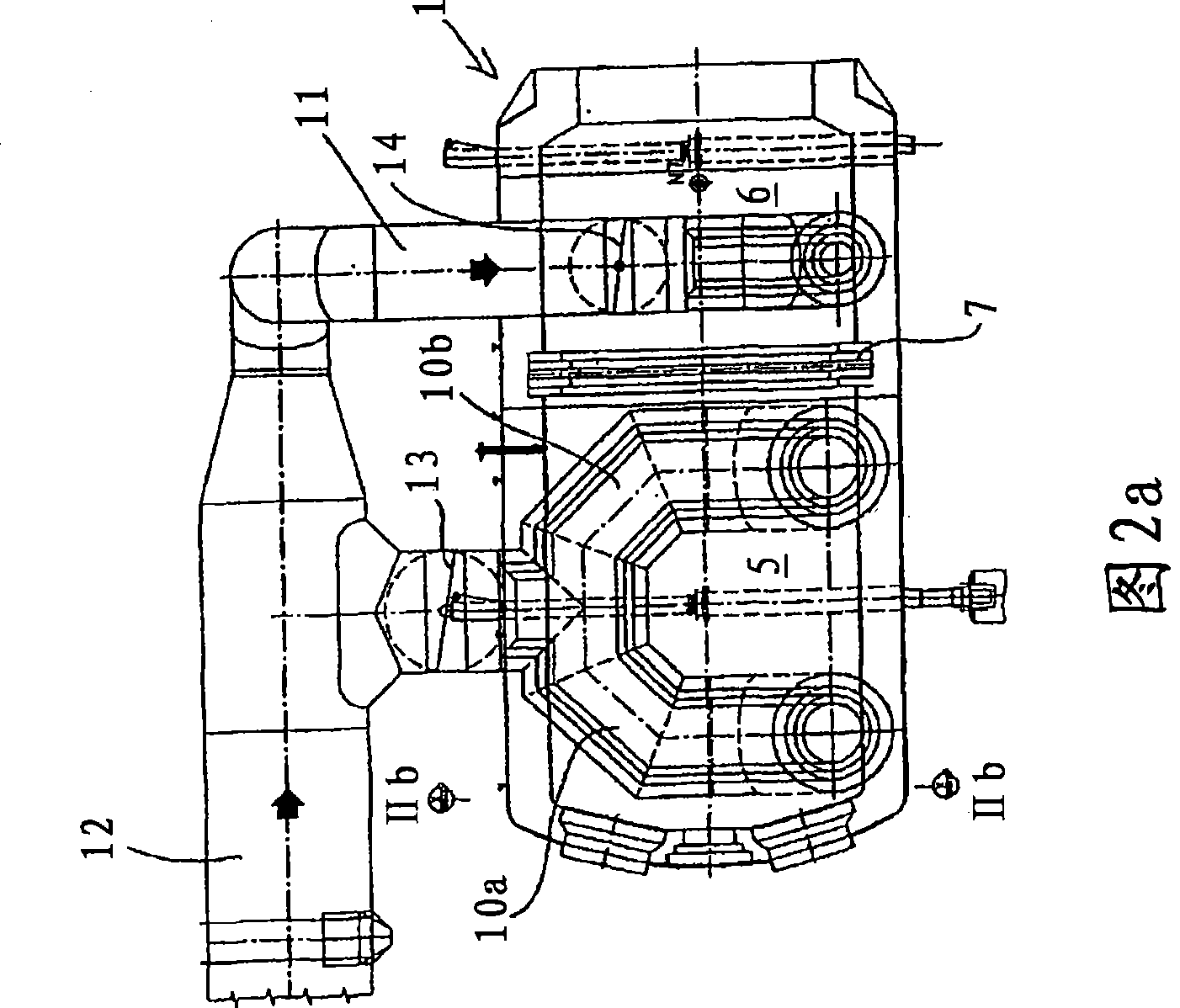
[0059] Fig. 9 shows a process flow diagram of the present invention through which a sulfur dioxide-containing gas having a sulfur dioxide concentration of 12% by volume is obtained. For this process, each of the first to fourth embodiments of the sulfur combustion furnace described with reference to FIGS. 1-5 can be used.
[0060] Provide 60.04 tons of sulfur per hour to the combustion furnace 1, with the help of 14,030Nm 3 Primary air at 120°C / h introduces sulfur into the furnace in atomized form. 175,000Nm to the first section 5 of the furnace 3 / h is filled with ambient air at 120°C, and 152,518Nm is supplied to the second part 6 of the furnace 3 / h Provide ambient air at 120°C. The residence time in furnace 1 was less than 0.6 s in total and less than 0.2 s in the second part.
[0061] In the first section 5, a sulfur dioxide concentration of 20.5% is obtained at a combustion temperature of 1,700-1,800°C, while at the outlet of the second section 6 or at the inlet of t...
Embodiment 2
[0063] In the flow chart of the second experiment shown in Fig. 10, the conditions in the first part 5 of the furnace 1 were identical to those in the first embodiment. However, the supply of combustion air at the outlet of the second section 6 is reduced to 38,669Nm 3 / h, so that the sulfur dioxide concentration obtained at the outlet of section 6 is 18%. This ensures that all sulfur vapor entering the second section 6 through the first section 5 is consumed either at the outlet of the second section 6 or at the inlet of the waste heat boiler 15 . The temperature of the gas stream when entering the waste heat boiler 15 is about 1,600°C. The temperature drops rapidly in the waste heat boiler 15 and falls below 1,000° C. in less than 0.5 s. Therefore, the retention time at high temperature is so short that no large amounts of nitrogen oxides are formed.
[0064]Since sulfur dioxide with a concentration of 18% by volume is not suitable for entering conventional sulfuric acid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com