Methods of treating influenza viral infections
A technology for effective treatment of influenza virus infection, applied in pharmaceutical formulations, medical preparations containing active ingredients, active ingredients of halogenated hydrocarbons, etc., can solve problems such as harmfulness, incomplete subtypes, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0119] Preparation of catecholbutane:
[0120] The catecholbutanes of the present invention can be prepared by any conventional method. For example, such compounds can be prepared as follows: U.S. Patent 5,008,294 (Jordan et al., issued April 16, 1991); U.S. Patent 6,291,524 (Huang et al., issued September 18, 2001); Hwu et al. (Hwu et al., "Antiviral activity of methylated nordihydroguaiaretic acids. 1. Synthesis, structural recognition, and inhibition of Tat-regulated HIV transactivation." Journal of Medicinal Chemistry ( J.Med.Chem) 4 / (16):2994-3000" (1998)); or McDonald et al., (McDonald, R.W. et al., Synthesis and Antibiotics of Dihydroguaiaretic Acid (NDGA) and Analogues Cancer activity." Anti-Cancer Drug Des., 16:261-270 (2001)).
[0121] In one embodiment of the invention, a catecholbutane, tetra-O-methyl NDGA, also known as meso-1,4-bis(3,4-dimethoxyphenyl)- 2,3-Dimethylbutane, terameprocol, EM-1421 or M4N (shown in the formula below) was prepared by preparing a re...
Embodiment 1
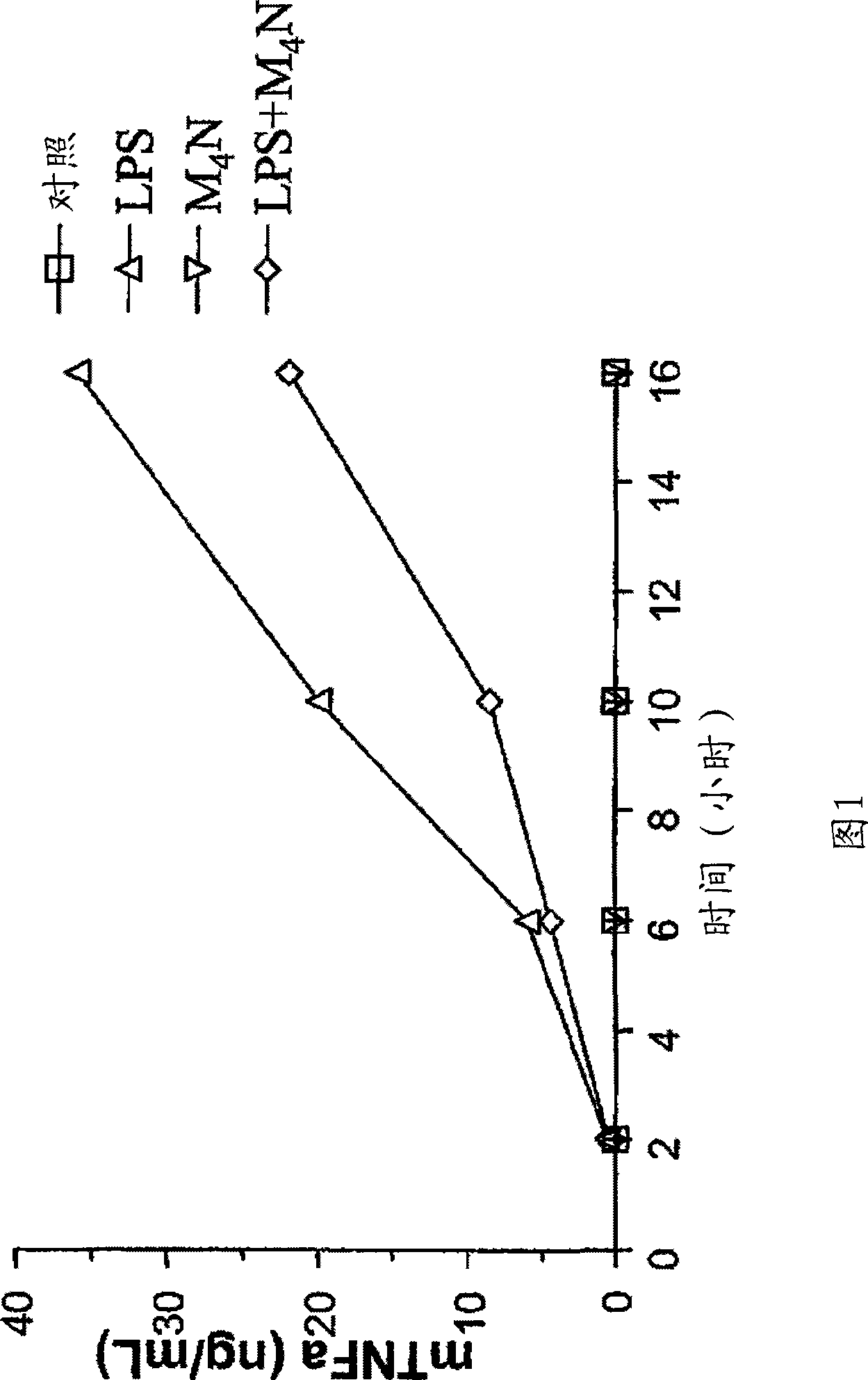
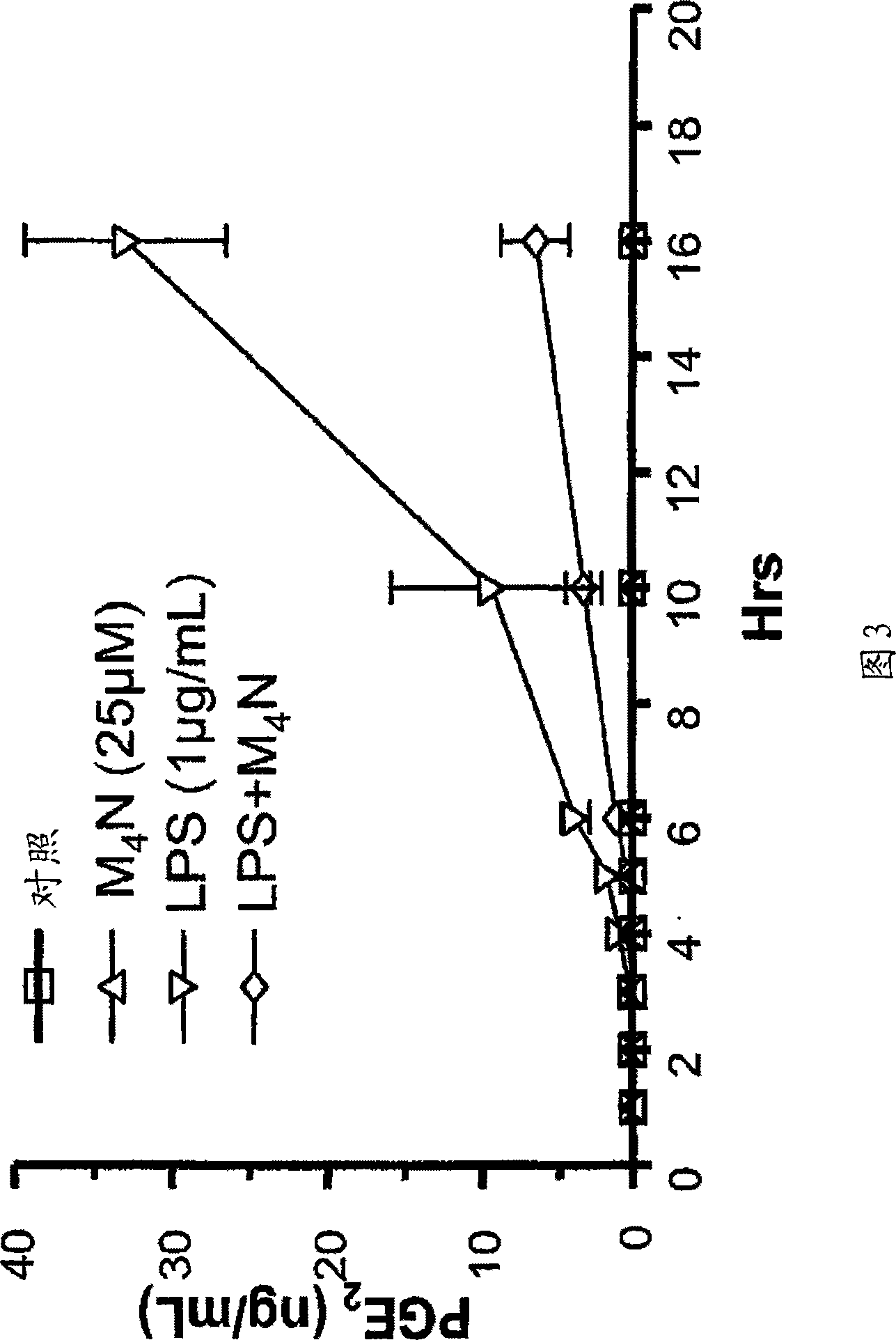
[0229] Catecholbutanes of general formula (I), ie M 4 Effect of N on TNF-α production by LPS-induced RAW 264.7 macrophages to determine M 4 N inhibits the ability of TNF-α induction. The effect of any catecholbutane of general formula (I) on the production of any pro-inflammatory cytokine in any LPS-stimulated macrophages can be determined using a method similar to that of this example.
[0230] As shown in Figure 1 and described below, M 4 N inhibited LPS-induced TNF-α overexpression in RAW 264.7 macrophages with a maximum inhibition of 57% at 10 h after induction.
[0231] More specifically, regarding the determination of M 4 N method to inhibit TNF-α induction by LPS, or leave 1.5 x 10 5 macrophages without treatment (control) or with LPS (1 μg / ml), M 4 N (25 μM) or both compounds were incubated at different time points. RAW 264.7 cells are mouse mononuclear macrophages. The LPS used was derived from Salmonella minnesota R595, which is available from List Biological ...
Embodiment 2
[0236] Catecholbutanes of general formula (I), ie M 4 Effect of N on TNF-α-induced apoptosis in murine fibroblasts to determine M 4 N inhibits the ability of TNF-α to induce apoptosis. The effect of any catecholbutane of general formula (I) on TNF-α-induced apoptosis in any type of cells can be determined using a method similar to that of this example.
[0237] Influenza infection induces the production of TNF-[alpha], and TNF-[alpha] pro-apoptotic activity is well known. Influenza viruses require apoptosis for efficient replication, and blocking TNF-α-induced apoptosis can reduce influenza virus replication and disease.
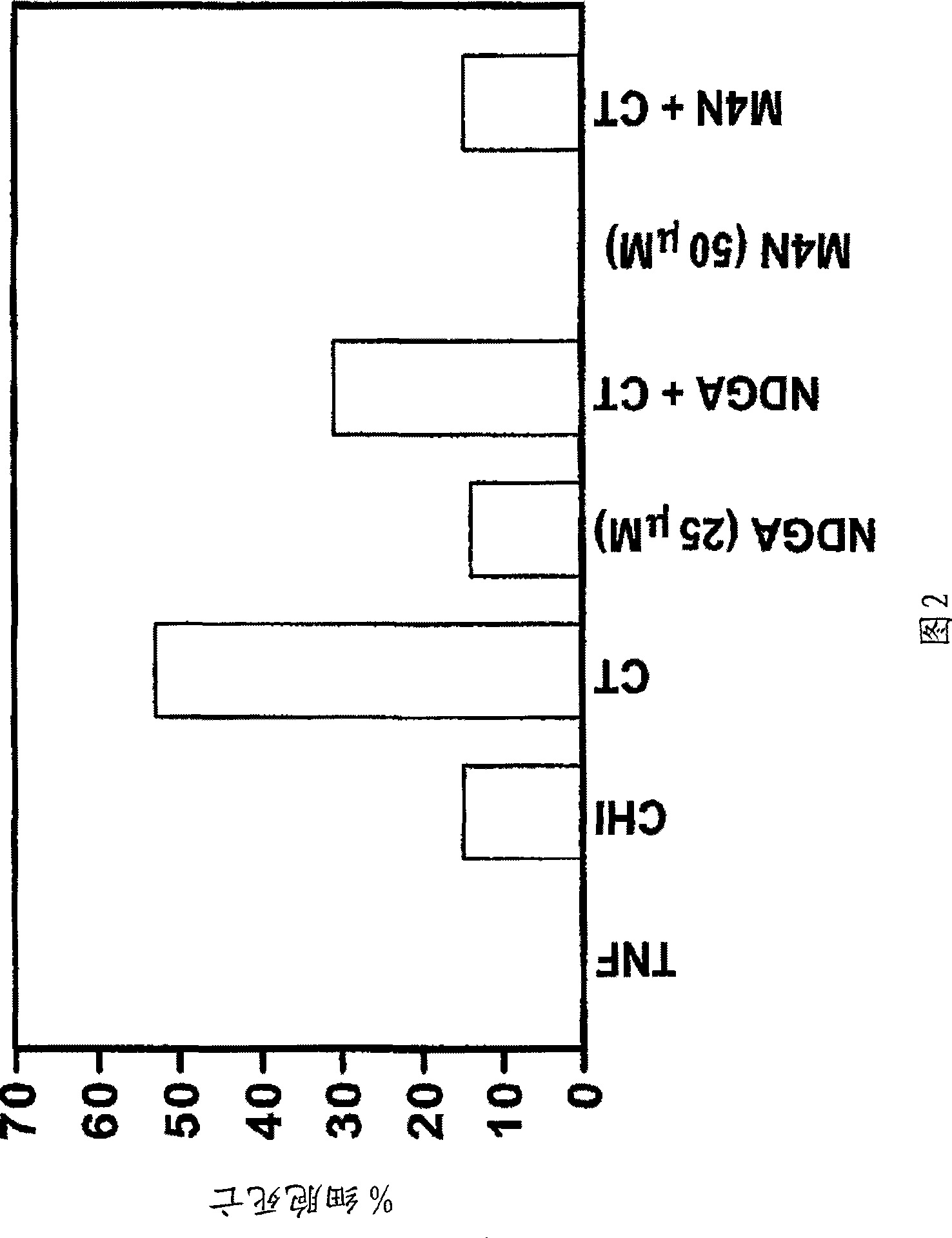
[0238] As shown in Figure 2 and described below, M4N strongly inhibited TNF-α-induced apoptosis in TNF-sensitive cells by cycloheximide. C3HA mouse fibroblasts in the absence / presence of NDGA (25 μM) or M 4 N (50 μM) was incubated with human recombinant TNF-α (20 ng / ml), cycloheximide (CHI) (10 ng / ml) or both. All compounds were added simultaneously and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com