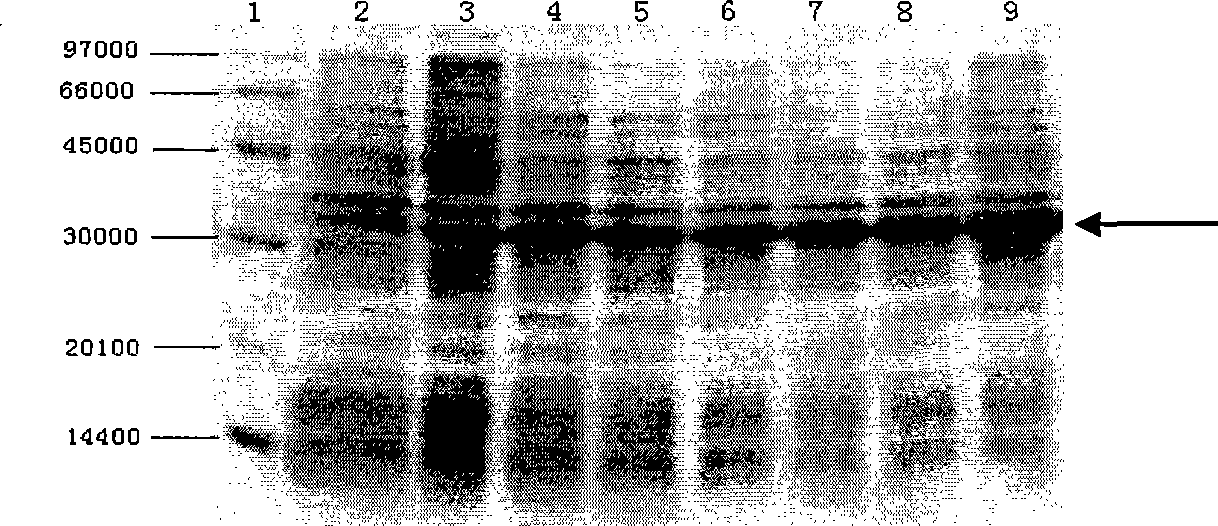Medicine capable of reducing uric acid content in blood
A drug and uric acid technology, applied in the field of drugs for reducing the concentration of uric acid in blood, can solve problems such as reducing the therapeutic effect, and achieve the effects of far-reaching social significance, high safety, and huge economic value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
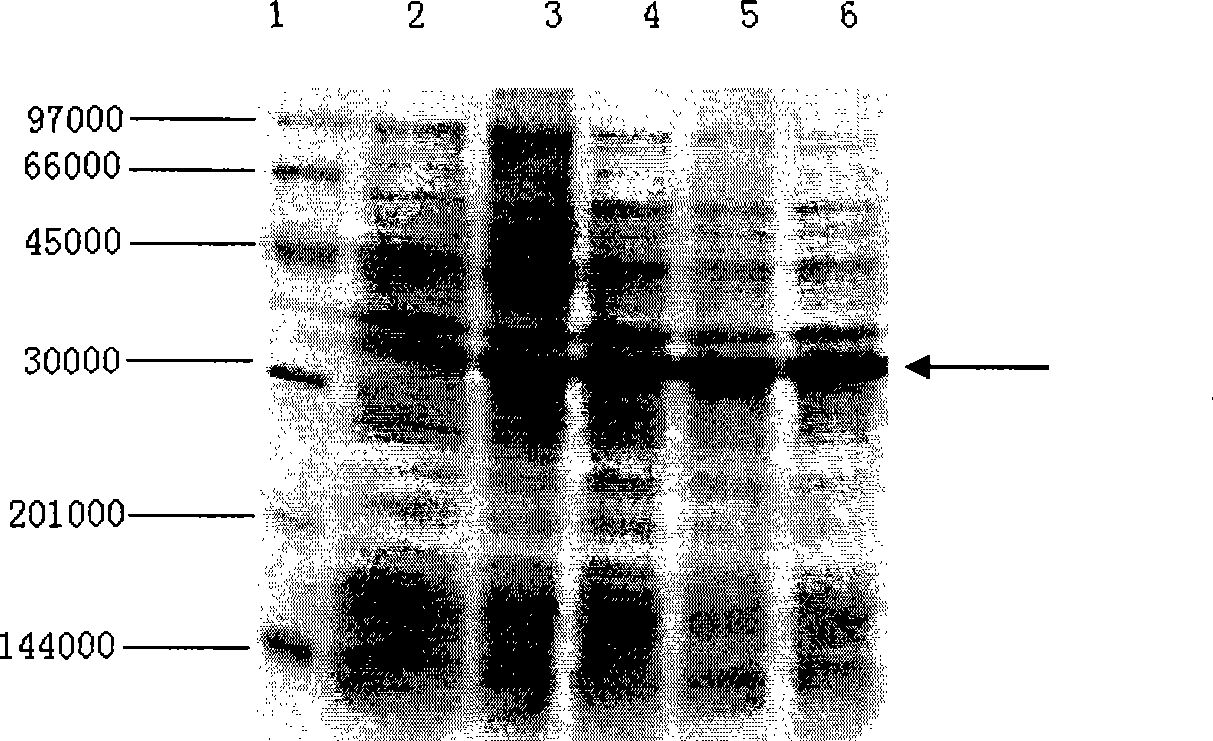
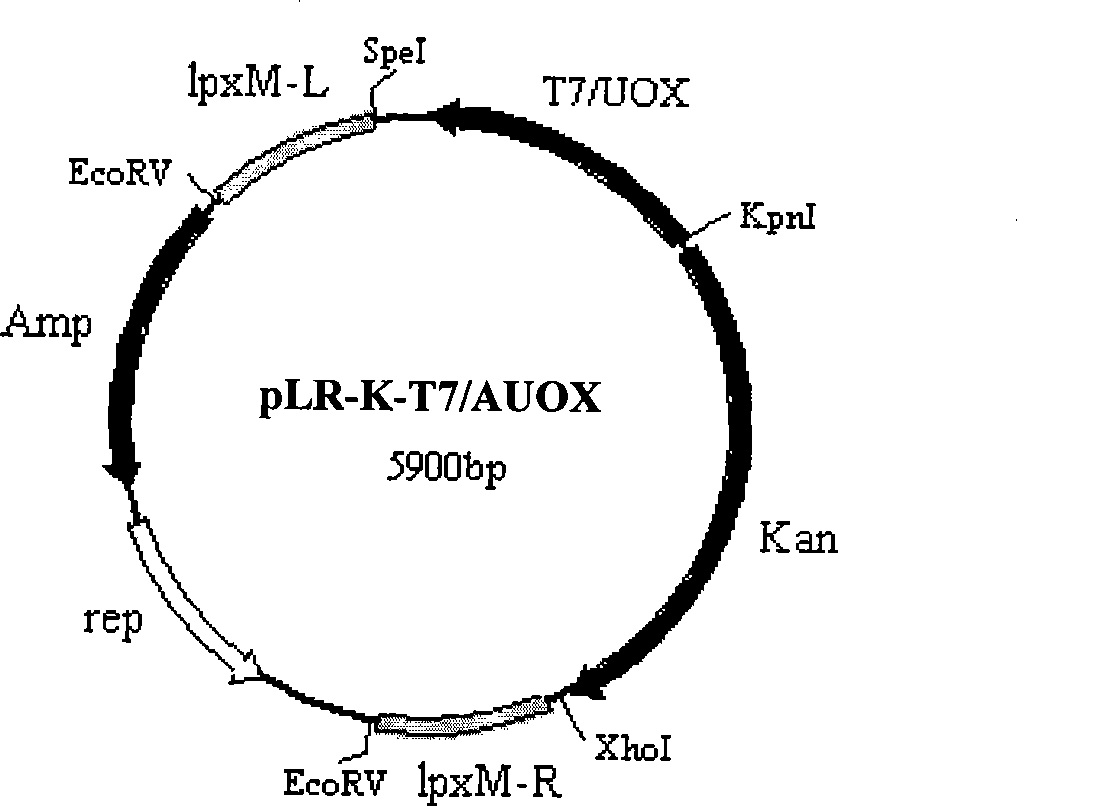
[0036] Embodiment 1, the construction of the engineering bacterium expressing urate oxidase
[0037] 1. Acquisition of urate oxidase gene (UOX)
[0038] (1) Acquisition of AUOX gene
[0039] 1. Genomic DNA extraction
[0040] Under sterile conditions, scrape the mycelium from the slant culture medium of Aspergillus flavus 3.1398 (China Common Microorganism Culture Collection Management Center) and insert it into 5ml Czapek's medium (sucrose 30g / L, NaNO 3 3g / L, MgSO4 ·7H 2 O 0.5g / L, KCl 0.5g / L, FeSO 4 ·7H 2 O 0.01g / L, K 2 HPO 4 ·2H 2 O 1.0g / L, peptone 15g / L; pH6.0-6.5), cultured at 30°C for 5 days. Take 2ml of the bacterial liquid to collect the flocculent bacterial cells by centrifugation, wash the precipitate once with sterile water; put the bacterial cells into a sterile mortar, pour about 20ml of liquid nitrogen into it for quick freezing, and grind the bacterial cells quickly when the liquid nitrogen has just evaporated. into a powder form; add 200 μl of yeast ly...
Embodiment 2
[0111] Embodiment 2, prepare microcapsule medicine with engineering bacterium
[0112] One, prepare microcapsule medicine with engineering bacteria I
[0113] (1) Formaldehyde inactivation
[0114] 1. Effect of formaldehyde inactivation on the ability of engineering bacteria to degrade uric acid
[0115] The selection of formaldehyde inactivation conditions refers to the "China Biological Products Regulations" 2000 edition (page 110). The final concentration of formaldehyde is generally between 1% (volume ratio) and 2% (volume ratio). The formaldehyde of % (volume ratio) and 2% (volume ratio) deactivates engineering bacteria I, and concrete steps are as follows:
[0116] Put 1 gram of wet bacteria in 25ml formaldehyde-physiological saline solution system (1% or 2% formaldehyde concentration), shake at 30°C or 37°C (200 rpm) for 30min. Samples were taken at the beginning, 10min, 15min, 20min, and 30min to determine the ability of the bacteria to degrade uric acid and the rep...
Embodiment 3
[0139] Embodiment 3, the property determination of microcapsule medicine
[0140] The experiments in this embodiment were all repeated three times, and the results were averaged.
[0141] 1. Determination of properties of microcapsule drug A
[0142] 1. The mechanical stability of the drug in gastrointestinal fluid
[0143] The prepared microcapsule drug A of Example 2 was respectively placed in normal saline, artificial gastric juice (dilute hydrochloric acid 16.4ml, add about 800ml of water and pepsin 10g, after shaking up, add water and release to 100ml), artificial intestinal juice ( Take 6.8g of potassium dihydrogen phosphate, add 500ml of water to dissolve, adjust the pH value to 6.8 with 0.1mol / L sodium hydroxide solution, take 10g of trypsin, add water to dissolve, mix the two liquids, add water to dilute to 1000ml) , 37°C, oscillating at 200 rpm, sampling every 24 hours, detecting microcapsule rupture in each solution with a microscope and taking pictures.
[0144]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com