EV-71 virus seed, inactivated vaccine for human and method of producing the same
An inactivated vaccine, EV-71 technology, applied in biochemical equipment and methods, antiviral agents, viruses/phages, etc., can solve the problems of non-specific vaccine application, achieve good growth and replication ability, and effective immunogenicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
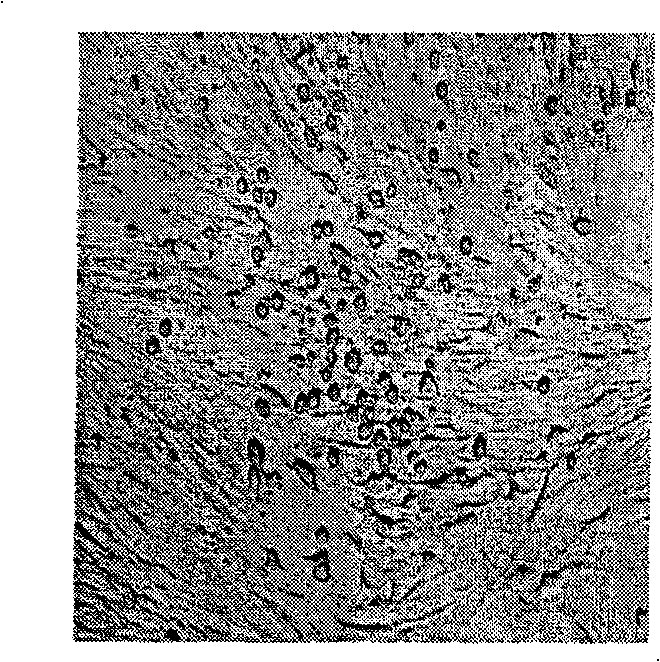
[0038] ——Collection and identification of EV-71 inactivated vaccine virus FY-23 virus
[0039] The secretions taken from the cotton swabs of the respiratory tract of critically ill patients with HFMD were inoculated in Vero cells, and then the same cells were subcultured twice, and then replanted with KMB-17 cells to grow to the seventh generation. Take 1ml of the virus liquid, perform RT-PCR according to the conventional method, obtain the cDNA sequence of the viral gene in segments, and connect the fragment into the puc-18 vector according to the conventional method, perform conventional nucleotide sequencing analysis, and use this sequence in the The results were confirmed by comparing with similar gene pools. Secondly, use anti-EV-71 specific serum, after diluting 1:4, mix it with 10-fold dilution of the virus in equal amounts, place it at 37°C for 1 hour, then inoculate Vero cells or KMB17 cells, and observe the lesion after culturing at 37°C for one week As a result, an...
Embodiment 2
[0041] The KMB-17 cell line was inoculated with the suspension adsorption method of the 15th generation working seed of the EV-71 vaccine virus, and cultured and grown at 37°C for 5 days. Analysis and purification, content determination, relevant dilution ratio, the vaccine prepared by adding aluminum hydroxide adsorption, consists of the following components: EV-71 inactivated purified antigen 100μg / ml, aluminum hydroxide 0.8-1mg / ml, thimerosal 0.05-0.1ng / ml.
Embodiment 3
[0043] ——Suspension adsorption method in the preparation process of EV-71 inactivated vaccine
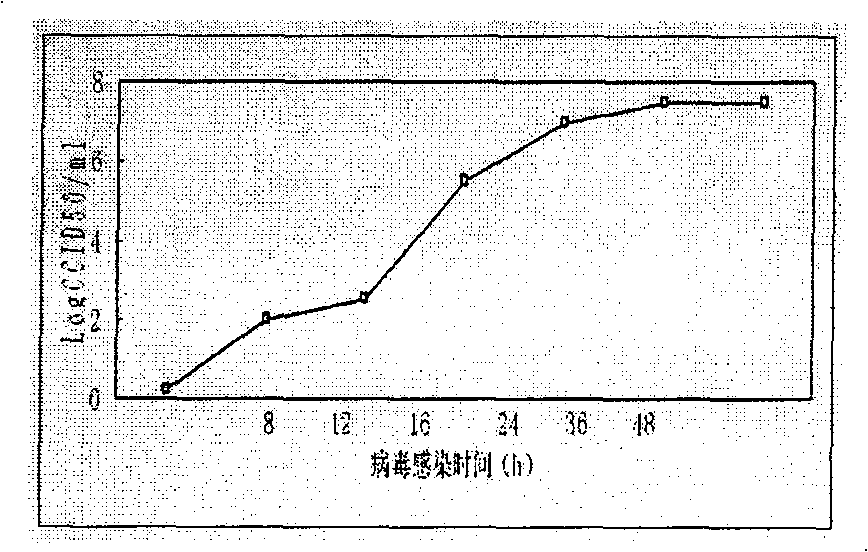
[0044] Take densely growing single-layer KMB-17 cells, digest them with 0.1% trypsin, remove the digestive juice, suspend the cells at 5% of the volume of the original culture medium, add 0.2-0.5ml virus solution, the moi is 0.05, mix well Incubate at 37°C for 30 minutes and shake gently in it, then transfer the suspension into a culture bottle, add an appropriate amount of growth medium, and culture at 37°C until the cells adhere to the wall and the lesions are completely harvested.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com