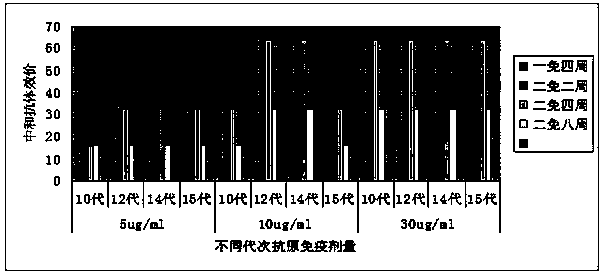
CoxA16 virus strain and human CoxA16 inactivated vaccine
An inactivated vaccine, coxa16 technology, applied in the direction of antiviral agents, viruses/phages, biochemical equipment and methods, etc., can solve the problems of non-specific vaccine application, and achieve good immunogenicity, safety, and high safety , effective immunogenic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of CoxA16 Inactivated Vaccine Virus Strain GX-20K-D
[0032] (1) The preparation method of CoxA16 inactivated vaccine virus strain GX-20K-D, comprising the following steps:
[0033] 1. The CoxA16 virus strain isolated from patients with hand, foot and mouth disease, as for the patient's throat swab and a small amount of herpes secretion, diluted with sterile PBS solution and filtered, inoculated in a single layer of KMB17 cells by suspension adsorption. 10 days after the lesion occurred, the KMB17 cells continued to be passaged for 4 passages. First, take 1ml of the virus liquid, perform RT-PCR according to the conventional method, obtain the cDNA sequence of the viral gene in segments, and connect the fragment into the puc-18 vector according to the conventional method, perform conventional nucleotide sequencing analysis, and use the sequence The results were confirmed by comparison in similar gene pools.
[0034] 2. The virus strain was ino...
Embodiment 2
[0045] Example 2 Preparation of CoxA16 Inactivated Vaccine
[0046] The preparation method of the CoxA16 inactivated vaccine of the present embodiment comprises the following steps:
[0047] 1. According to the KMB17 cell production and verification regulations, select the 28th generation KMB17 cells with dense growth, discard the culture medium, digest the cells with 0.1% trypsin, discard the trypsin solution, and use 4% of the volume of the original culture medium, DMEM Suspension cells.
[0048] 2. Add the 14th generation CoxA16 virus solution of the present invention to the above cell suspension, add 0.2-0.5ml of the virus solution, the moi is 0.02-0.1, mix well and incubate at 37°C for 30 minutes, and add light Shake, then transfer the suspension into a culture bottle, add an appropriate amount of cell growth medium, culture at 37°C for 3 to 4 days, and harvest after the cells adhere to the wall and complete lesions appear.
[0049] 3. Add formaldehyde to the virus ha...
Embodiment 3
[0061] Example 3 Safety Testing of CoxA16 Inactivated Vaccine Finished Products
[0062] The finished vaccine is injected into the peritoneal cavity of 18-20g mice with a weight of 0.5ml / mouse, a total of 20, and there should be no death within one month (routine abnormal toxicity test: 20 mice with a weight of 18-20g are injected with 0.5ml / mouse of the finished vaccine) Mice, fed for 7 days, without death, weight gain (about 1-10 grams); 2 guinea pigs, intraperitoneal injection of 5ml vaccine finished products, feeding for 7 days, no death, weight gain (about 1-40 grams) was judged as Qualified), the weight gain of the mice should be the same as that of the normal saline control group (see Table 2).
[0063]
[0064] The formaldehyde content of the finished vaccine should not be higher than 50μg / dose, and the bacterial endotoxin should not be higher than 100EU / dose. Should be negative in sterility testing (see Table 3).
[0065]
[0066] Organization Applicant
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com