Method for preparing ferrocenyl cyclic amine compound sensing material and use
A sensing material, ferrocene technology, applied in the field of sensing material preparation, can solve the problem of slow progress in anion recognition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
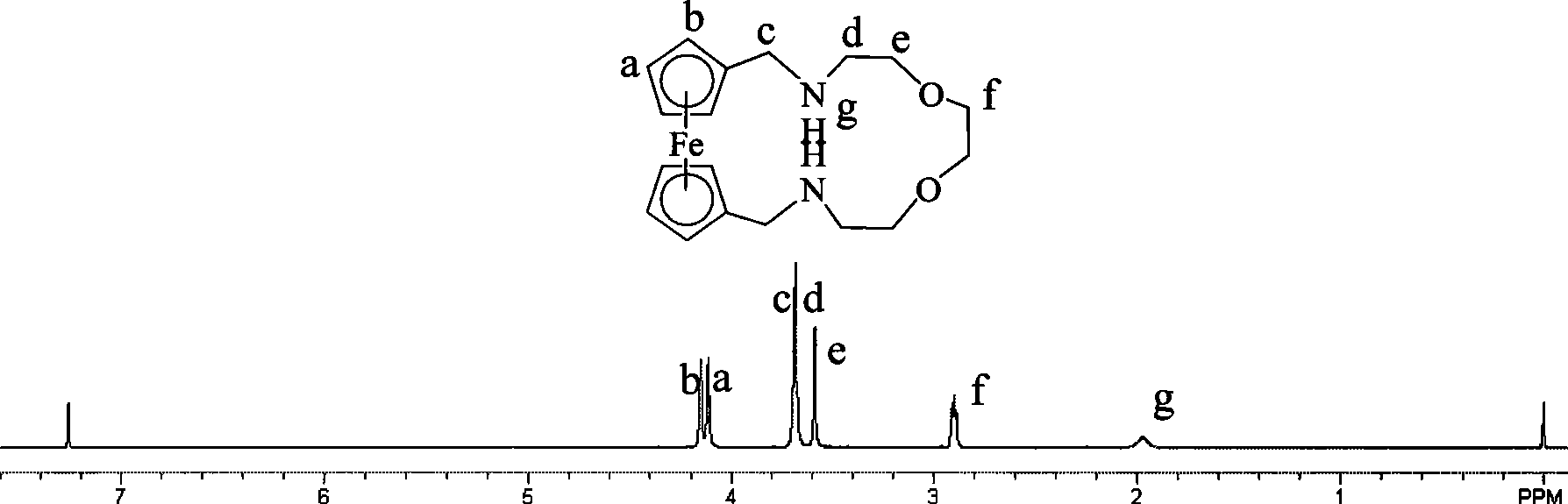
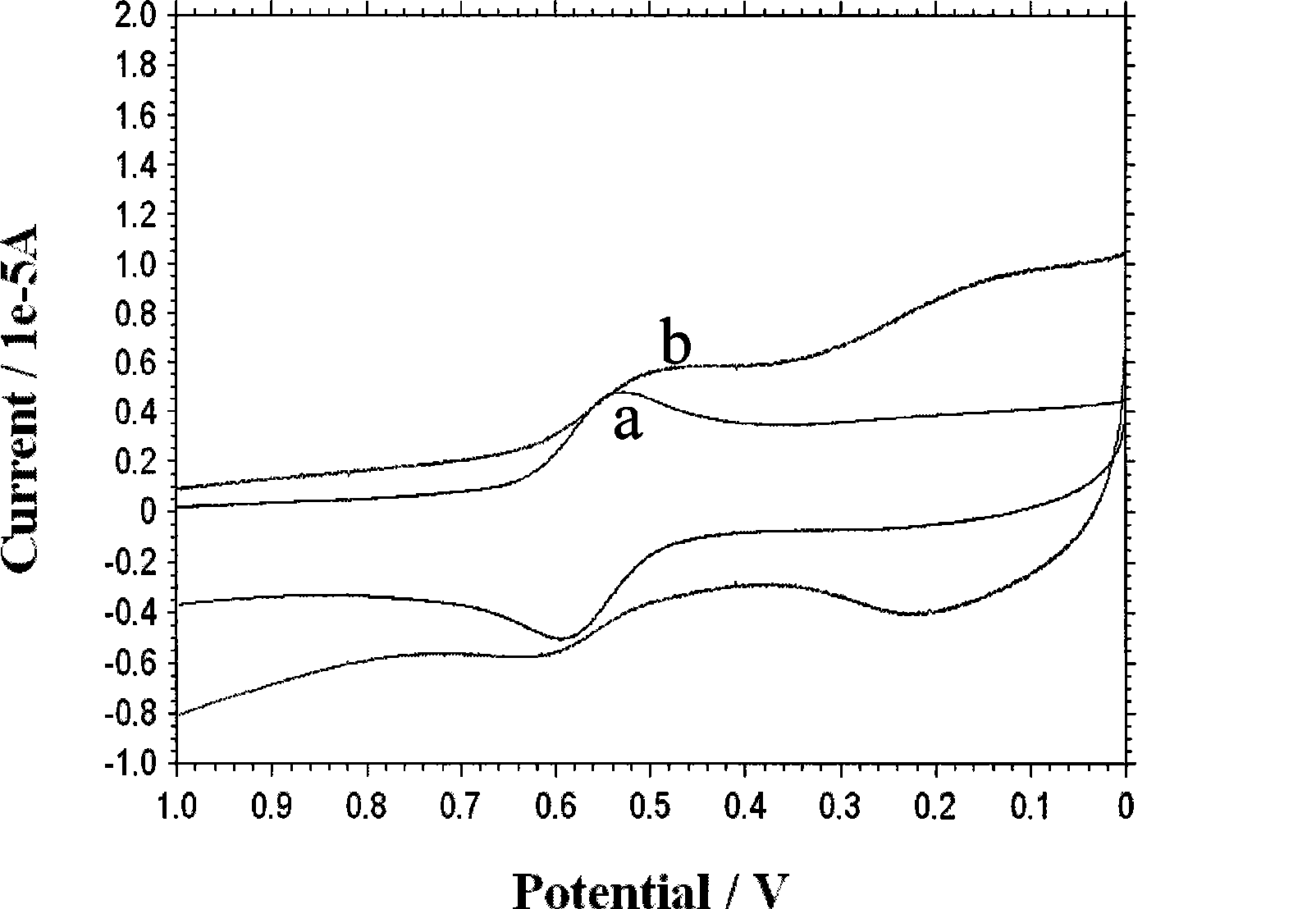
[0030] Synthesis of ferrocenedicarbaldehyde (G.G.A. Balavoine, G. Doisneau, et al, [J]. J. Organometal, Chem., 1991, 412, 381-382;). Take 0.5g (2.1mmol) ferrocenediformaldehyde and dissolve it in 20mLCH 3 In CN, take 0.3mL 1,2-bis(2-aminoethoxy)ethane (2.1mmol) and dissolve it in 20mL CH 3 CN, then 20 mL of ferrocenedicarbaldehyde in acetonitrile and 20 mL of 1,2-bis(2-aminoethoxy)ethane in CH 3 The CN solution was slowly added dropwise to 50mL acetonitrile solution, and the drop was completed within 1h. The reaction was protected from light at room temperature for 48h. Filtration, and the filtrate was evaporated to dryness to obtain a red viscous liquid. Then dissolve the red viscous liquid with 50mL methanol, add 0.5gNaBH in two 4 (13.1mmol), reacted at room temperature for 2h, and stopped the reaction. The solvent was evaporated to dryness under reduced pressure, dissolved in 30 mL of dichloromethane, and inorganic salts were filtered off. Dichloromethane was evaporat...
Embodiment 2
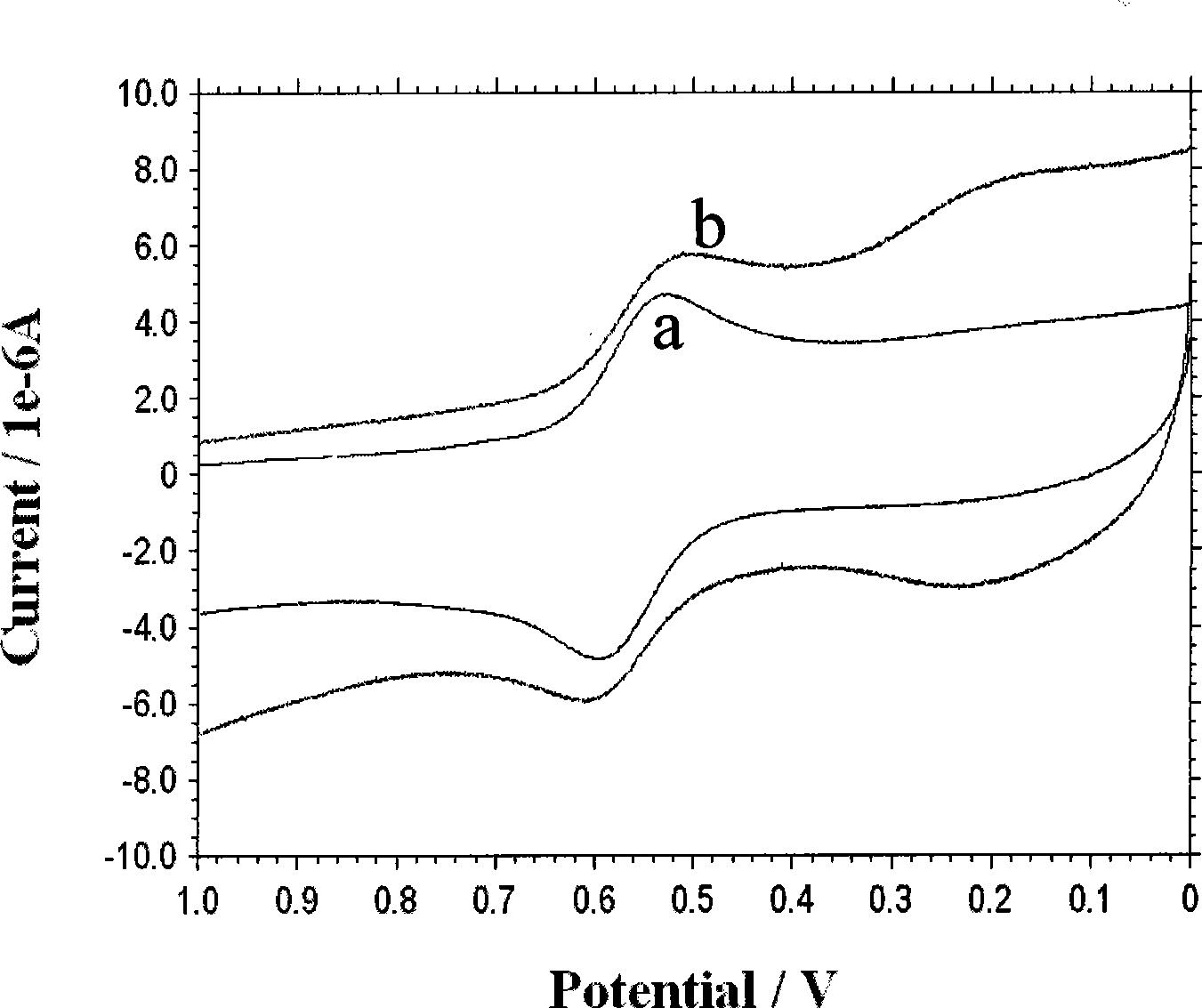
[0033] Synthesis of ferrocenedicarbaldehyde (G.G.A. Balavoine, G. Doisneau, et al, [J]. J. Organometal, Chem., 1991, 412, 381-382;). Take 0.25g (1.05mmol) ferrocenediformaldehyde and dissolve it in 10mLCH 3 In CN, take 0.15mL 1,2-bis(2-aminoethoxy)ethane (1.05mmol) and dissolve it in 10mL CH 3 CN, then 10 mL of ferrocenedicarbaldehyde in acetonitrile and 1,2-bis(2-aminoethoxy)ethane in 10 mL of CH 3 The CN solution was slowly added dropwise to 25mL acetonitrile solution, and the drop was completed within 1h. The reaction was protected from light at room temperature for 48h. Filtration, and the filtrate was evaporated to dryness to obtain a red viscous liquid. Then dissolve the red viscous liquid with 25mL methanol, add 0.25gNaBH in two 4 (6.55mmol), reacted at room temperature for 2h, and stopped the reaction. The solvent was evaporated to dryness under reduced pressure, then dissolved in 15 mL of dichloromethane, and the inorganic salt was filtered off. Dichloromethane ...
Embodiment 3
[0036] Synthesis of ferrocenedicarbaldehyde (G.G.A. Balavoine, G. Doisneau, et al, [J]. J. Organometal, Chem., 1991, 412, 381-382;). Take 1.0g (4.2mmol) ferrocenedicarbaldehyde dissolved in 40mL CH 3In CN, take 0.6mL 1,2-bis(2-aminoethoxy)ethane (4.2mmol) and dissolve it in 40CH 3 CN, then 40 mL of ferrocenedicarbaldehyde in acetonitrile and 1,2-bis(2-aminoethoxy)ethane in 40 mL of CH 3 The CN solution was slowly added dropwise to 100mL acetonitrile solution, and the drop was completed within 1h. The reaction was protected from light at room temperature for 48h. Filtration, and the filtrate was evaporated to dryness to obtain a red viscous liquid. Then dissolve the red viscous liquid with 100mL methanol, add 1.0gNaBH in two 4 (26.32mmol), reacted at room temperature for 2h, and stopped the reaction. The solvent was evaporated to dryness under reduced pressure, then dissolved in 60 mL of dichloromethane, and the inorganic salt was filtered off. Dichloromethane was evapora...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com