Method for preparing swine fever recombinant subunit vaccine
A subunit vaccine, swine fever technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, recombinant DNA technology, etc., can solve the problem of limited number of antigenic epitopes and affecting immune effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
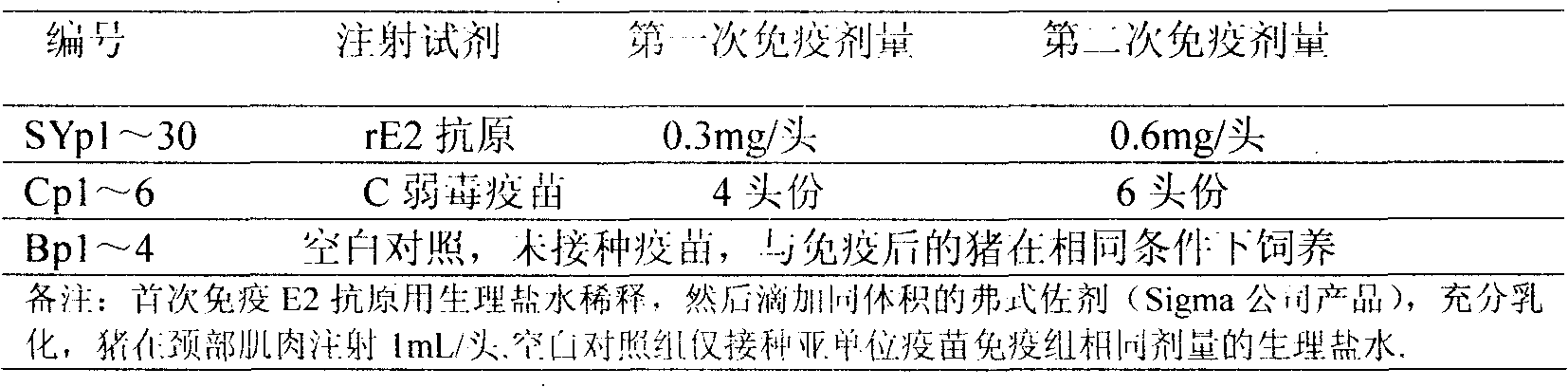
[0032] 1. Cloning of E2 gene antigen region and construction of recombinant expression vector pCSFV-rE2
[0033] According to the method for amplifying the CSFV E2 gene, two pairs of specific primers containing restriction enzyme sites were designed, as shown in Table 1. The sequence span of a single E2 gene is 569 bp, and after concatenation, it is 1138 bp.
[0034] Table 1: Primer number, sequence, position, length
[0035]
[0036]
[0037] Note: Primer E2-2F: GAATCC Restriction site sequence; GGC...TTC amino acid linker sequence; ATG...GAT is the original sequence of E2 gene; RBS sequence
[0038] The total RNA of the vaccine strain (C-strain) is extracted, and the main antigen region fragment of the E2 gene is amplified by RT-PCR. Double-digest the pGEX-6p-1 vector and the purified E2 fragment, connect the two genes using the common EcoR I restriction site, construct a recombinant expression vector, and transform it into DH5α competent cells. The construction s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com