Composite capable of strengthening anti-tumor immune response
An anti-tumor immunity and compound technology, applied in the field of biomedicine, can solve problems such as limitations, low application efficiency, and poor specificity, and achieve the effect of up-regulating anti-tumor immune response ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Design and screening of Foxp3 siRNA
[0031] Find the sequence through NCBI and design siRNA using ambion design software. The siRNA of the design sequence was obtained by chemical synthesis, and 4 segments of Foxp3 specific siRNA were synthesized, and Foxp3 siRNA with stable expression and good interference effect was screened out: the inventor designed and synthesized image 3 The Foxp3 siRNA as shown, and these Foxp3 siRNA were transfected into Treg separated by magnetic beads (stemcell, Canada) by liposome (lipo2000, invitrogen, USA) in an in vitro experiment [9,21] , Through real-time quantitative PCR and flow cytometry to detect the degradation of Foxp3 mRNA [9,21] , From which a Foxp3 siRNA (siRNA3, Figure 4 A). After transfecting 4 segments of Foxp3 specific siRNA into Treg, RT-PCR and realtime-PCR were used to evaluate the specific degradation of Foxp3 siRNA on Foxp3 mRNA. The study found that in a series of synthesized siRNA, the degradation efficiency ...
Embodiment 2
[0049] Example 2 Evaluation of Foxp3 siRNA transfection efficiency via liposome and analysis of its effect on Treg cell apoptosis:
[0050] Different concentrations (5nM, 10nM, 20nM) of Foxp3 siRNA were transfected into Treg cells by liposome (lipo2000, invitrogen, USA), and the transfection efficiency was evaluated with FITC fluorescently-labeled siRNA. At the same time, the flow cytometer (FACSCalibur , BD Company, USA) analyzed the effect of different concentrations of Foxp3 siRNA transfection on Treg cell apoptosis. The study found that the transfection efficiency of Foxp3 siRNA can reach 41.7% ( Figure 5 B). At the same time, about 15% of cells undergo early apoptosis or death ( Figure 5 A), while the transfection efficiency of 5nM or 10nM Foxp3 siRNA is only 17% and 25%. In view of the above results, the inventor of the present application used 20nM as the optimal transfection concentration of Foxp3 siRNA in subsequent studies.
Embodiment 3
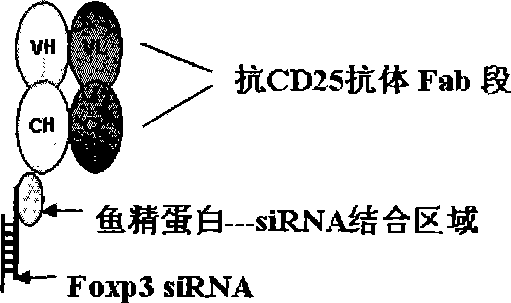
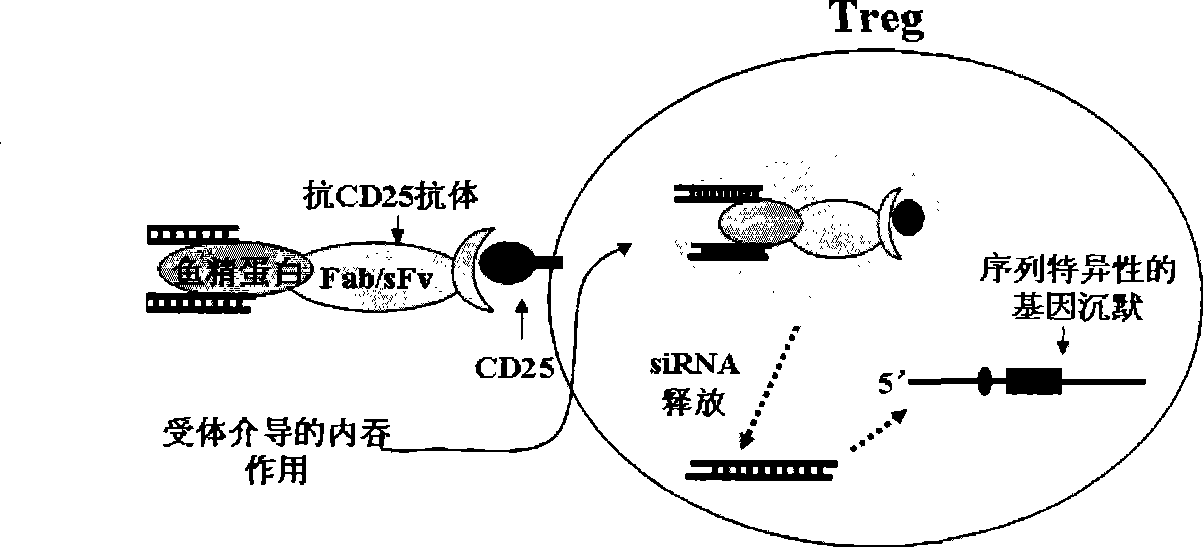
[0051] Example 3 Preparation of Foxp3 siRNA-protamine-anti-CD25 antibody complex
[0052] The baculovirus expression system was used to express His-tagged anti-CD25-scFv monoclonal antibodies and scFv-protamine fragments (amino acids 8-29, scFv-P), and purified by high performance liquid chromatography to obtain purified proteins 5mg (combined by Beijing Yixinxingye Company). The synthesized Foxp3 siRNA was mixed with anti-CD25-scFv monoclonal antibody and scFv-protamine fragment in PBS buffer at a molar ratio of 6:1:1. After 30 minutes at 4 degrees Celsius, the Foxp3 siRNA was completed -Binding of protamine-anti-CD25 antibody complex.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com