Crosslinkable silicone coating compositions
A technology of polysiloxane and composition, applied in the direction of coating, devices for coating liquid on the surface, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment and
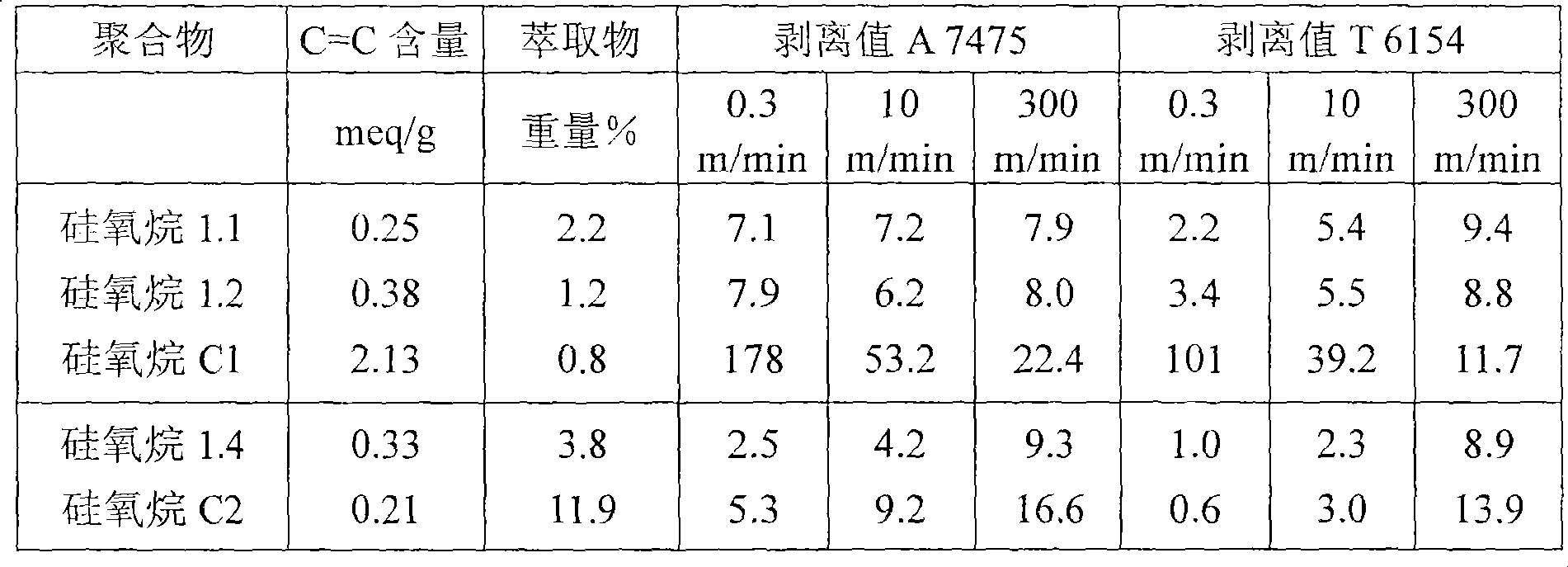
[0129] Examples and Comparative Experiments :
[0130] Use a mixture of the following ingredients as a standard formulation:
[0131]100 parts by weight of various polymer siloxanes 1.1 to 1.4 according to the invention and comparative polymer siloxanes C1 to C4 with trimethylsiloxane end units and a viscosity of 25 mPa·s (25° C.) Straight-chain hydromethylsiloxane, the amount that ensures the molar ratio of SiH groups to unsaturated groups of various polymers is 1.6:1,
[0132] 1 part by weight of platinum-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex with a viscosity of 1000 mpa·s at 25°C in α,ω-diethylene A solution at a concentration of 1% by weight (based on elemental platinum) in dimethicone, and
[0133] 0.25 parts by weight of 1-ethynylcyclohexanol.
[0134] These mixtures are used to coat paper.
[0135] As a substrate, a paper with the trade name Glassine Silca Classic from the company Ahlstrom was used. Coating was carried out at 60 m / min on a Dixon coati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com