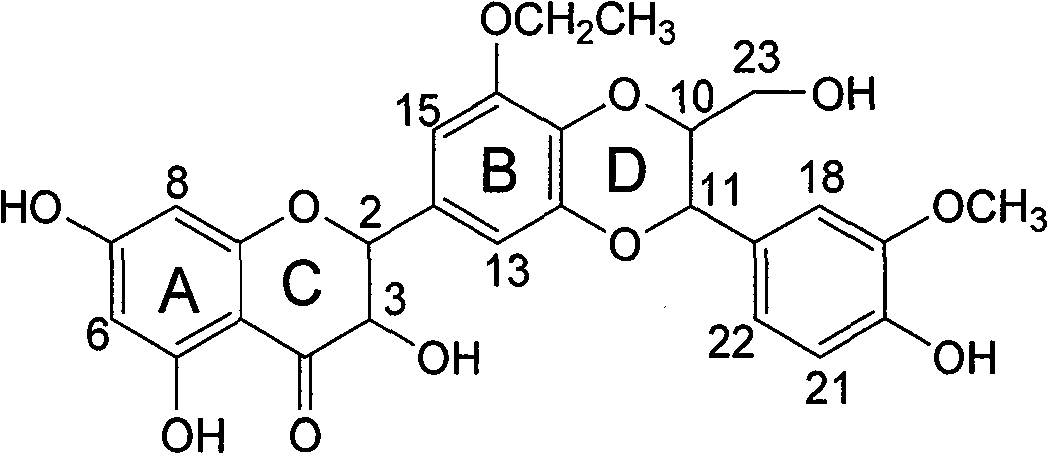
Pharmacy use of ethyoxyl substituted silybin for inhibiting herpes simplex virus
An ethoxy and purpose technology, which is applied in the field of pharmaceutical use of ethoxy to replace silibinin to inhibit herpes simplex virus, can solve problems such as not being effectively developed, and achieves simple synthesis route, high yield, and is beneficial to industrial production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
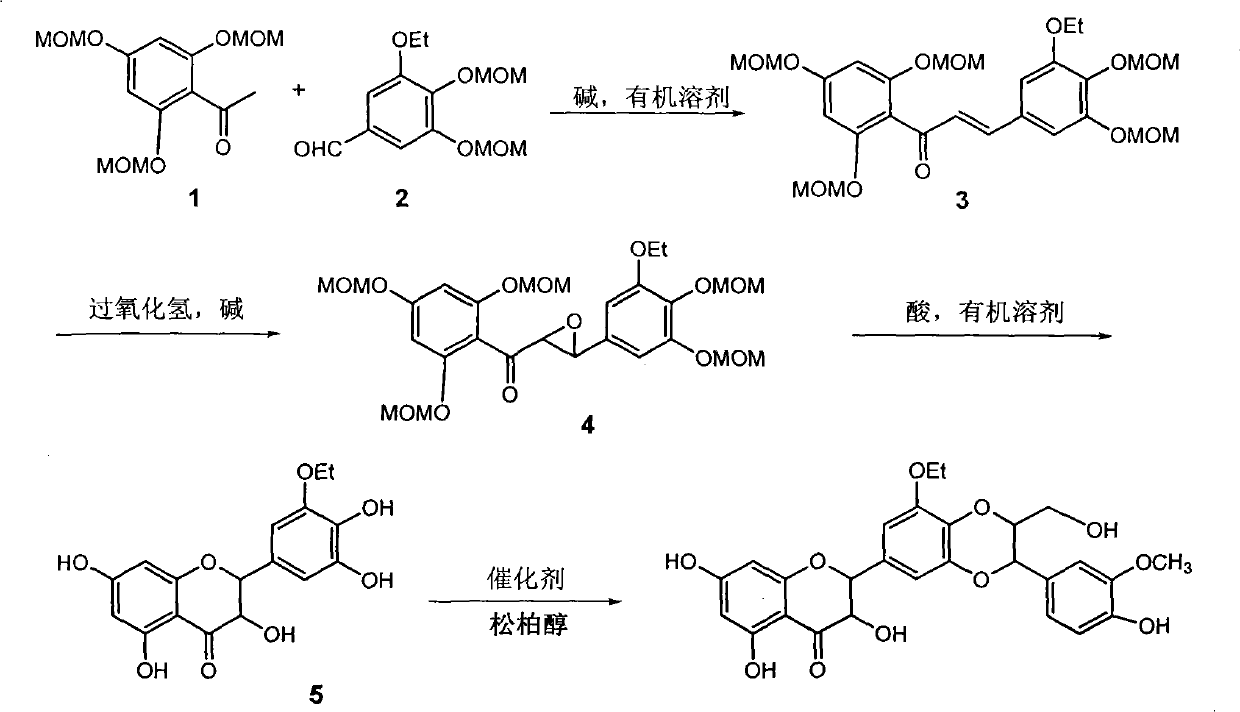
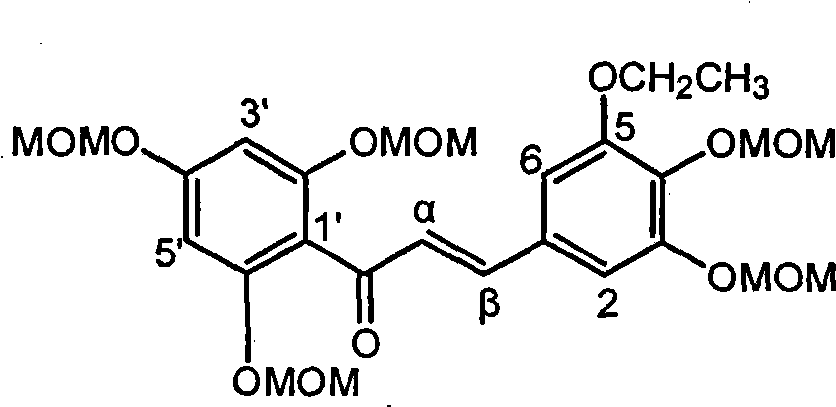
[0018] Example 1 : the preparation of compound 3 [1-(2,4,6-trimethoxymethoxyphenyl)-3-(3,4-dimethoxymethoxy-5-ethoxyphenyl) propenone]
[0019]
[0020] 3 grams of 3-ethoxy-4,5-dimethoxymethoxybenzaldehyde and 3.5 grams of 2,4,6-trimethoxymethoxyacetophenone were dissolved in 40 milliliters of ethanol, and dissolved with 10 grams of hydrogen A 20 ml aqueous solution of potassium oxide was stirred at room temperature for 15 hours. Ethanol was distilled off under reduced pressure, 30 ml of water was added, and extracted with ethyl acetate (3×20 ml). After combining the organic phases, they were washed successively with saturated sodium bisulfite (40 ml) and saturated brine (40 ml), dried over anhydrous sodium sulfate, concentrated by filtration, and obtained compound 3[1-(2,4, 4.4 g of 6-trimethoxymethoxyphenyl)-3-(3,4-dimethoxymethoxy-5-ethoxyphenyl)propenone] yellow oil, yield 70%. R f (petroleum ether / ethyl acetate=2 / 1): 0.40; UV (methanol) λ max =210,325nm; H NMR spe...
Embodiment 2
[0021] Example 2 : Compound 4 [1-(2,4,6-trimethoxymethoxyphenyl)-3-(3,4-dimethoxymethoxy-5-ethoxyphenyl) oxide acetone]
[0022]
[0023] Get the compound 1-(2,4,6-trimethoxymethoxyphenyl)-3-(3,4-dimethoxymethoxy-5-ethoxyphenyl) propenone obtained in Example 1 Dissolve 3 g in 50 ml of methanol, add 4 ml of 2N sodium hydroxide and 4 ml of 30% hydrogen peroxide under stirring, stir at room temperature for 10 hours, evaporate the solvent under reduced pressure, add 50 ml of water, and extract with ethyl acetate (3 x 30 ml). The organic phases were combined, washed with saturated brine (40 mL), and dried over anhydrous sodium sulfate. Filtration and concentration gave 2.6 g of a yellow oily substance, with a yield of 85%.
[0024] 1-(2,4,6-trimethoxymethoxyphenyl)-3-(3,4-dimethoxymethoxy-5-ethoxyphenyl)epoxyacetone: R f (petroleum ether / ethyl acetate=2 / 1): 0.40; UV (methanol) λ max =206,281nm; H NMR spectrum 1 H NMR (400MHz, deuterated chloroform) δ: 1.39 (triplet, J=7.2...
Embodiment 3
[0025] Example 3 : Preparation of compound 5 [(±) 5'-ethoxyl-3,5,7,3',4'-pentahydroxydihydroflavone]:
[0026]
[0027] 2.8 g of compound 4 [1-(2,4,6-trimethoxymethoxyphenyl)-3-(3,4-dimethoxymethoxy-5-ethoxyphenyl) glycidone] dissolved In 35 milliliters of methanol and 15 milliliters of tetrahydrofuran, add 5 milliliters of concentrated hydrochloric acid dropwise, stir at 50-55 ° C for 30 minutes, evaporate the solvent under reduced pressure, extract with ethyl acetate (3 × 30 milliliters), combine the organic phases and use Wash with saturated brine (40 ml), and dry over anhydrous sodium sulfate. After filtration, concentration, and column chromatography, 0.75 g of yellow powder was obtained, with a yield of 45%.
[0028] R f (chloroform / methanol=10 / 1): 0.15; H NMR spectrum 1 H NMR (400MHz, deuterated acetone) δ: 1.33 (triplet, J=7.2Hz, 3H, CH 3 ), 4.08 (quartet, J=7.2Hz, 2H, CH 2 ), 4.62 (double doublet, J=12.0, 4.0Hz, 1H, H-3), 4.68 (doublet, J=4.0Hz, 1H, 3-OH), 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com