Oral solid preparation of Febuxostat with high-bioavailability and preparation method thereof
A technology of febuxostat and solid preparations, which is applied in the mixed crystal form and amorphous micronized tablet and its preparation, and in the field of febuxostat single crystal form, which can solve the problem of high preparation and not enough to ensure the preparation of bioavailability And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
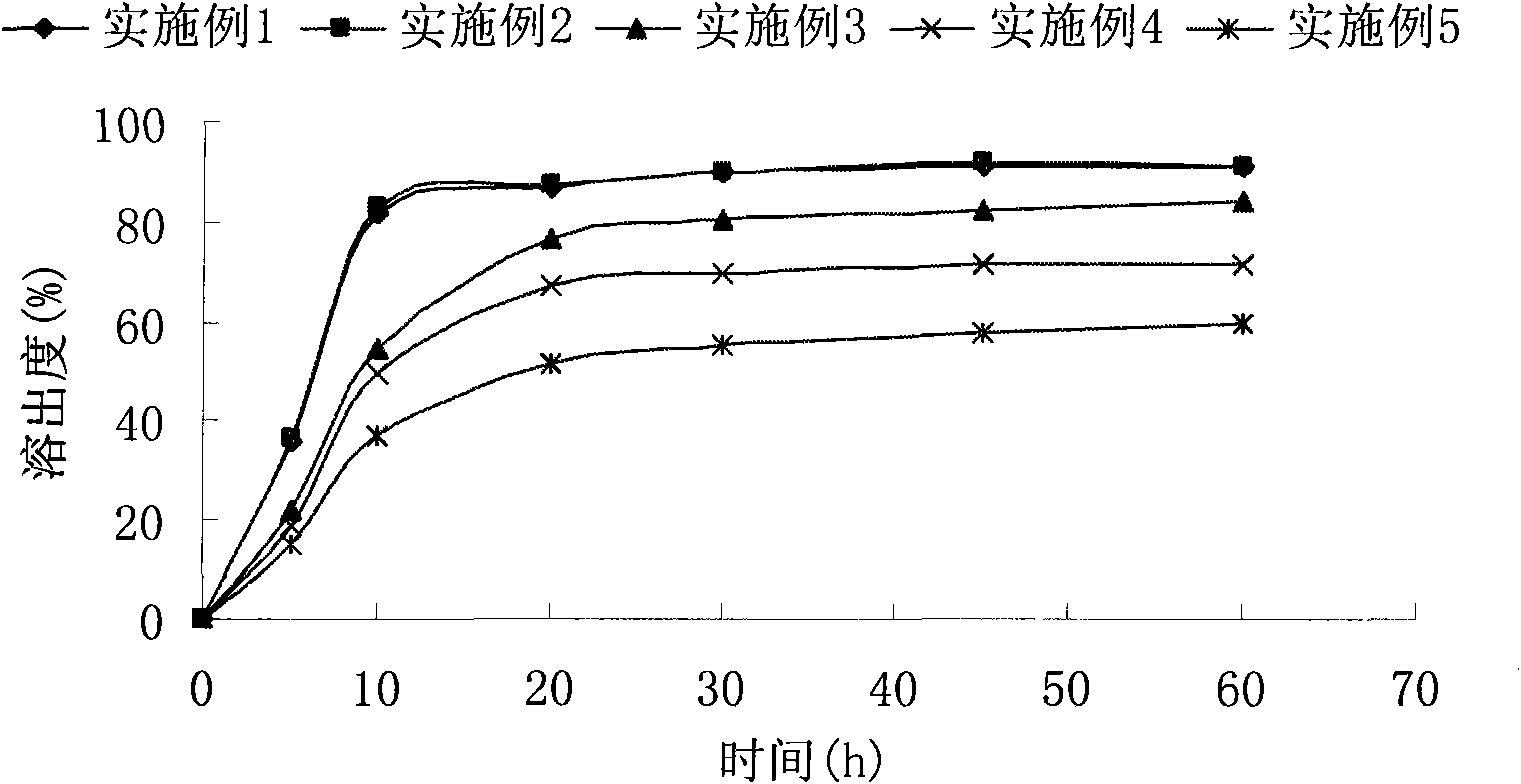
Examples
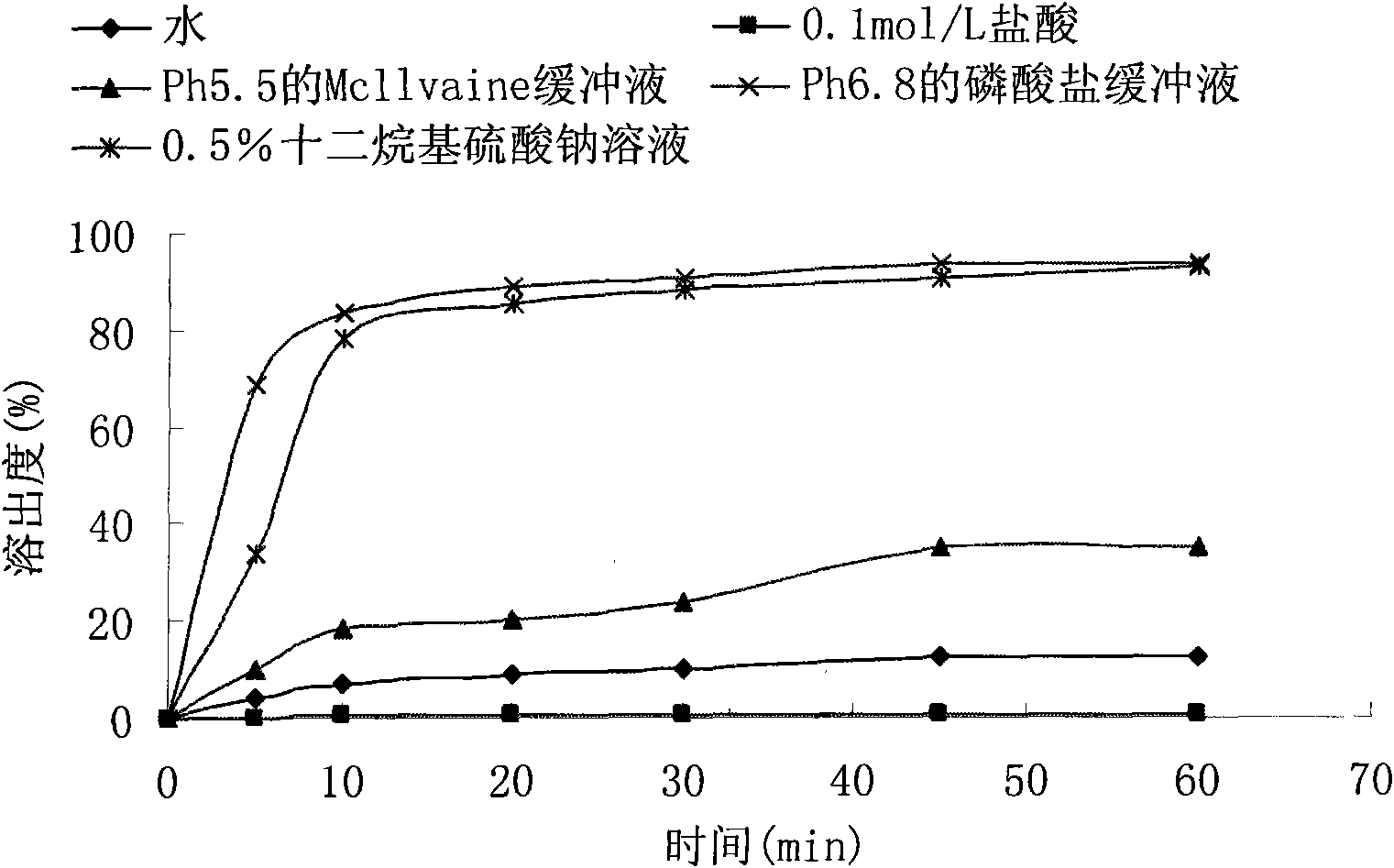
Embodiment 1
[0053] prescription:
[0054] Febuxostat (H crystal form) 120mg
[0055] Lactose 240mg
[0056] Microcrystalline cellulose 60mg
[0057] Croscarmellose Sodium 15mg
[0058] Magnesium stearate 4.5mg
[0059] 1% hypromellose aqueous solution
[0060] Preparation Process:
[0061] 1. Treat the febuxostat raw material to an average particle size range of 1~3.5μm, and use it for later use;
[0062] 2. Pass the lactose, microcrystalline cellulose, croscarmellose sodium and magnesium stearate through an 80-mesh sieve for use;
[0063] 3. Mix hypromellose with purified water to a concentration of 1% for later use;
[0064] 4. Mix febuxostat, lactose, microcrystalline cellulose and croscarmellose sodium uniformly, add the binder to make the soft material, the soft material is sieved to make wet granules, and dried at 50℃~60℃, After the dried granules are sieved and granulated, magnesium stearate and croscarmellose sodium are added and mixed to obtain a semi-finished product. Determine the content of...
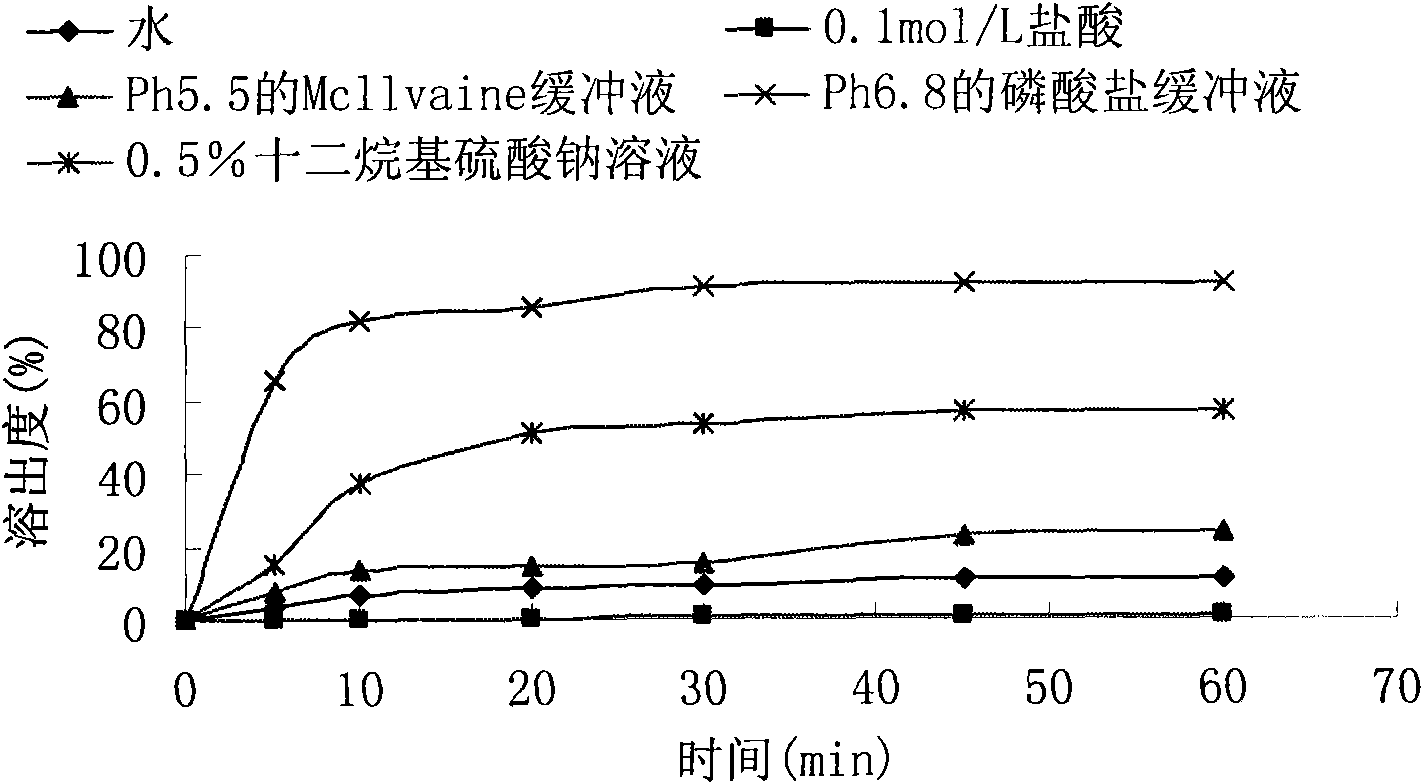
Embodiment 2
[0066] prescription:
[0067] Febuxostat (H crystal form) 120mg
[0068] Lactose 240mg
[0069] Microcrystalline cellulose 60mg
[0070] Croscarmellose Sodium 15mg
[0071] Magnesium stearate 4.5mg
[0072] 1% hypromellose aqueous solution
[0073] Preparation Process:
[0074] 1. Treat the febuxostat raw material to an average particle size range of 3.5-10μm for use;
[0075] 2. Pass the lactose, microcrystalline cellulose, croscarmellose sodium and magnesium stearate through an 80-mesh sieve for use;
[0076] 3. Mix hypromellose with purified water to a concentration of 1% for later use;
[0077] 4. Mix febuxostat, lactose, microcrystalline cellulose and croscarmellose sodium uniformly, add the binder to make the soft material, the soft material is sieved to make wet granules, and dried at 50℃~60℃, After the dried granules are sieved and granulated, magnesium stearate and croscarmellose sodium are added and mixed to obtain a semi-finished product. Determine the content of the semi-finishe...
Embodiment 3
[0079] prescription:
[0080] Febuxostat (H crystal form) 120mg
[0081] Lactose 240mg
[0082] Microcrystalline cellulose 60mg
[0083] Croscarmellose Sodium 15mg
[0084] Magnesium stearate 4.5mg
[0085] 1% hypromellose aqueous solution
[0086] Preparation Process:
[0087] 1. Treat the febuxostat raw material to an average particle size in the range of 10-20μm for use;
[0088] 2. Pass the lactose, microcrystalline cellulose, croscarmellose sodium and magnesium stearate through an 80-mesh sieve for use;
[0089] 3. Mix hypromellose with purified water to a concentration of 1% for later use;
[0090] 4. Mix febuxostat, lactose, microcrystalline cellulose and croscarmellose sodium uniformly, add the binder to make the soft material, the soft material is sieved to make wet granules, and dried at 50℃~60℃, After the dried granules are sieved and granulated, magnesium stearate and croscarmellose sodium are added and mixed to obtain a semi-finished product. Determine the content of the semi-f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com