Alpha 1-adrenalin receptor antagonist, and preparation and medical use thereof
A receptor antagonist, epinephrine technology, applied in the direction of pharmaceutical formulations, drug combinations, medical preparations containing active ingredients, etc., can solve the problems of not obtaining subtype selectivity prediction ability, not having α1B drugs on the market, etc., and achieving excellent Treatment of benign prostatic hyperplasia, long survival time, good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
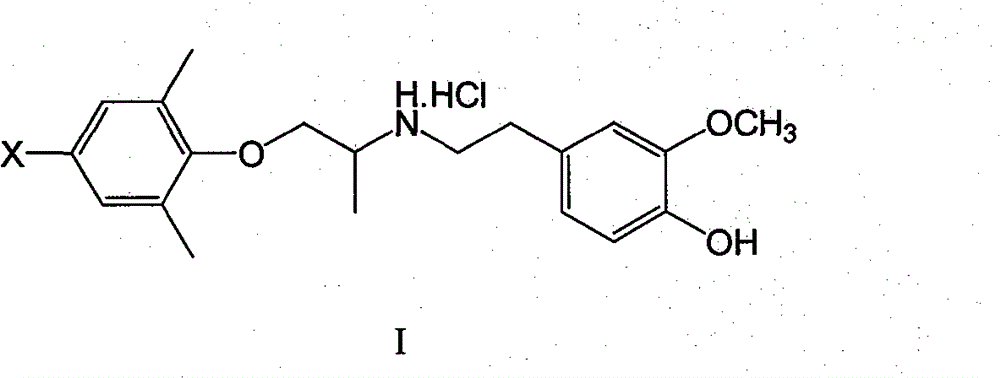
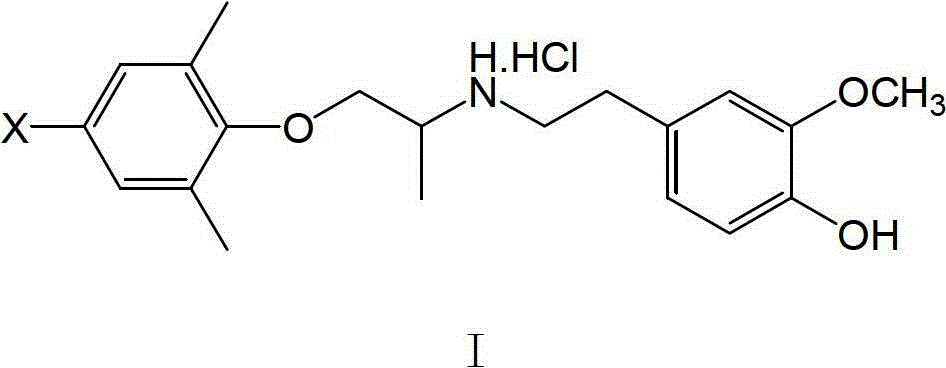
[0061] XBMI: 1-(2,6-Dimethyl-4-bromophenoxy)-2-(3-methoxy-4-hydroxy)propane hydrochloride
[0062] Dissolve 1.5mmol of 4-bromo-2,6-dimethylphenoxyacetone and 1.6mmo of 3-methoxy-4-hydroxyphenethylamine in 5ml of methanol, add a small amount of p-toluenesulfonic acid as a catalyst, and reflux 3h, lowered to room temperature, added 0.2g KBH in batches 4 , so that the temperature of the reaction solution does not exceed 30°C, and react at room temperature for 3h. The methanol was evaporated under reduced pressure, extracted with a small amount of ethyl acetate, the insoluble matter was filtered off, the ethyl acetate was evaporated, the residue was dissolved in absolute ether, and dry HCl gas was introduced. A white solid was obtained, mp 149.8-152.4°C, yield 35%.
[0063] IR (KBr, cm -1 ): 3420, 2926, 1605, 1524, 1279, 1207, 1032.
[0064] 1 HNMR (DMSO-D 6 )δ: 1.43(d, 3H, J=6.47Hz, CH CH 3 ), 2.26(s, 6H, 2×ArCH 3 ), 2.94 (m, 2H, ArCH 2 ), 3.21 (m, 2H, NCH 2 ), 3.63(m,...
Embodiment 2
[0068] XBM Ⅱ: 1-(2,4,6-trimethylphenoxy)-2-(3-methoxy-4-hydroxy)propane hydrochloride
[0069] The reaction conditions were the same as XBMI, and a white solid was obtained, mp 164-166°C, yield 36%.
[0070] IR (KBr, cm -1 ): 3427, 2934, 1610, 1524, 1269, 1217, 1034.
[0071] 1 HNMR (DMSO-D 6 )δ: 1.43 (d, J=6.70Hz, 3H, CH CH 3 ), 2.19 (s, 3H, ArCH 3 ), 2.22(s, 6H, 2×ArCH 3 ), 2.97 (m, 2H, ArCH 2 ), 3.21 (m, 2H, NCH 2 ), 3.63(m, 1H, CHN), 3.73~3.76(2s, 6H, 2×ArOCH 3 ), 3.91 (m, 2H, OCH 2 ), 6.29~6.93(m, 5H, ArH), 9.06(d, 2H, J=37.35Hz, NH 2 + ).
[0072] Molecular formula: C 21 h 29 NO 3 .HCl.
[0073] HRMS: [M+H] + Meas. Mass, 344.22145; Calc. Mass, 344.22202.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com