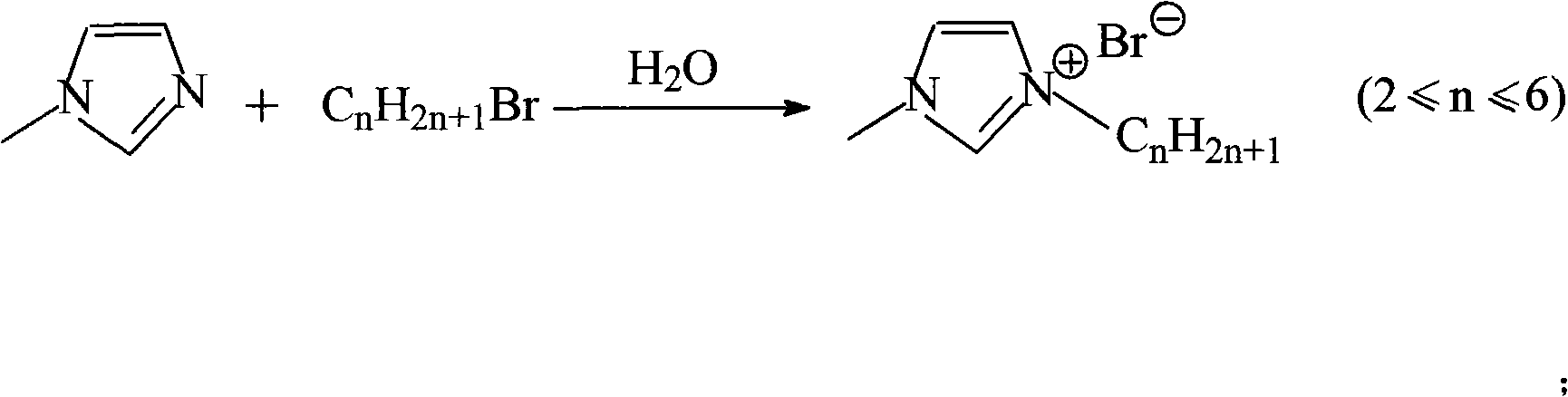
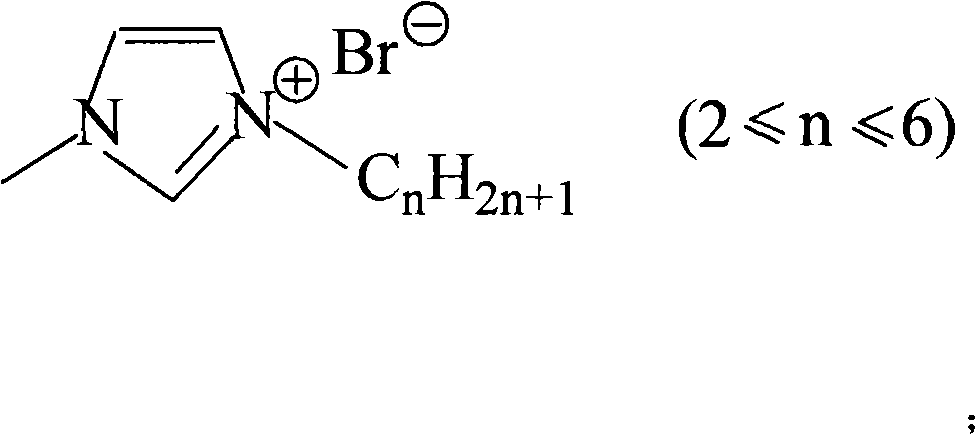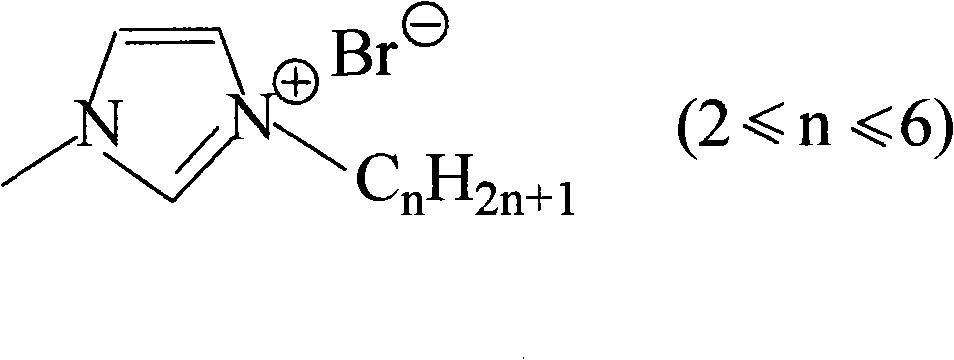Method for preparing achromatic imidazole ionic liquids
An ionic liquid and imidazole technology, which is applied in the field of preparation of colorless imidazole ionic liquids, can solve the problems of complex operation, high cost, inability to meet large-scale application requirements, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] When n=2, the bromoalkane is bromoethane. Mix ethyl bromide and 1-methylimidazole at a volume ratio of 1:1, add solvent water to it, the volume ratio of water to the above mixture is 0.2:1, and reflux at 40°C for 12 hours under nitrogen protection. The unreacted bromoethane in the lower layer was removed, and the water and unreacted bromoethane in the product were removed by vacuum distillation at 40°C for 10 h to obtain colorless 1-ethyl-3-methylimidazolium bromide.
Embodiment 2
[0014] When n=2, the bromoalkane is bromoethane. Mix ethyl bromide and 1-methylimidazole at a volume ratio of 3:1, add solvent water to it, the volume ratio of water to the above mixture is 0.2:1, and reflux at 40° C. for 12 hours under nitrogen protection. The unreacted bromoethane in the lower layer was removed, and the water and unreacted bromoethane in the product were removed by vacuum distillation at 40°C for 10 h to obtain colorless 1-ethyl-3-methylimidazolium bromide.
Embodiment 3
[0016] When n=2, the bromoalkane is bromoethane. Mix ethyl bromide and 1-methylimidazole at a volume ratio of 6:1, add solvent water to it, the volume ratio of water to the above mixture is 0.2:1, and reflux at 40° C. for 12 hours under nitrogen protection. The unreacted bromoethane in the lower layer was removed, and the water and unreacted bromoethane in the product were removed by vacuum distillation at 40°C for 10 h to obtain colorless 1-ethyl-3-methylimidazolium bromide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com